1 not a helix-slideshow
- 2. The Science and History of Topologically Non-Linked (“TN”) DNA
- 3. The Double Non-Helix The Science and History of Topologically Non-Linked (“TN”) DNA Ken Biegeleisen, M.D., Ph.D. kb@NotAHelix.net Part I By Copyright 2006, all rights reserved.
- 4. Biegeleisen, K. Topologically non-linked circular duplex DNA. Bull Math Biol, 64:589-609, 2002. Major journal reference:
- 5. The author is indebted generally to Mercury Computer Systems, and particularly to Mr. Patrick Barthelemy, for making available the AmiraMol virtual molecular modeling program, without which this work could not even have been started, much less finished. Acknowledgment
- 6. Introduction History Introduction to alkali denaturation of circular DNA Basic topology (helix-superhelix transition) Alkali denaturation according to “traditional” W-C theory Why TN DNA must be a right handed superhelix Alkali denaturation according to the TN theory RL helical transition Resistance of Form I to denaturation Form IV, structure and properties Experimental evidence, introduction The topology equation (Lk = T+W) EM studies of replicative intermediates Critical evaluation of Crick et al, “Is DNA really a double helix?” Critical evaluation of Stettler et al (work of Charles Weissmann) Chambers discovers “+” and “–” circular strands reanneal to Form I Tai Te Wu separates strands of Form I on agarose gels Conclusions References Table of Contents Click this button to start show:
- 7. Dr. Biegeleisen, c. 1972Dr. Biegeleisen, c. 1995 Previous slide Next slide Table Of Contents
- 8. Dr. Biegeleisen, c. 1995Wu R. and T.T. Wu (1996). A novel intact circular dsDNA supercoil. Bull. Math. Biol, 58:1171-1185. Previous slide Next slide Table Of Contents
- 9. (2006) Previous slide Next slide Table Of Contents
- 10. HISTORY Previous slide Next slide Table Of Contents
- 11. Previous slide Next slide Table Of Contents
- 12. (Is “A” in the front? Or is it in the back?) Previous slide Next slide Table Of Contents
- 13. Previous slide Next slide Table Of Contents
- 14. Cairns, J. (1963). The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 6, 208-213. Cairns, J. (1963). The chromosome of Escherichia coli. Cold Spring Harbor Sym Quant Biol. 28:43-46. Previous slide Next slide Table Of Contents
- 15. Previous slide Next slide Table Of Contents
- 16. Previous slide Next slide Table Of Contents
- 17. Previous slide Next slide Table Of Contents
- 18. Previous slide Next slide Table Of Contents
- 19. Previous slide Next slide Table Of Contents
- 20. Previous slide Next slide Table Of Contents
- 21. Wouldn’t this make more sense? Previous slide Next slide Table Of Contents
- 22. Two models for TN DNA (Topologically-Non-linked DNA) Previous slide Next slide Table Of Contents
- 23. Two models for TN DNA (Topologically-Non-linked DNA) Previous slide Next slide Table Of Contents
- 24. Although by 1963 it was established, to the satisfaction of nearly all, that DNA was a right-handed helix when stripped of all proteins, dried and crystallized, this hardly proved that it would have the same structure in the cell nucleus. The nucleus, after all, contains not only DNA, but also an approximately equal weight of highly-positively charged basic protein, which would surely exert some sort of effect on the conformation of the DNA.
- 25. Although by 1963 it was established, to the satisfaction of nearly all, that DNA was a right-handed helix when stripped of all proteins, dried and crystallized, this hardly proved that it would have the same structure in the cell nucleus. The nucleus, after all, contains not only DNA, but also an approximately equal weight of highly-positively charged basic protein, which would surely exert some sort of effect on the conformation of the DNA.
- 26. What did Cairns think? Previous slide Next slide Table Of Contents
- 27. A Turning Point In History Previous slide Next slide Table Of Contents Cairns invited to Cold Spring HarborSymposium on DNA structure and replicationNot yet known: strands of circular chromosomes don’t separate.Not discovered: enzymes capable of replicating circular DNASole fact: chromosomes are circular, and they do replicate.Most logical explanation: strands are topologically non-linked.Least likely explanation: countless twists unwound/rewound.
- 29. Previous slide Next slide Table Of Contents
- 30. What are the implications of the “swivel” theory? How fast is this molecule spinning? What are the implications of the “swivel” theory? How fast is this molecule spinning?
- 31. E. coli statistics: WHOLE CELL: Length = 2 ; width = 0.5 . CHROMOSOME: length = 1,354 = 1.35 mm! (The chromosome is 700x as long as the entire cell!) Molecular weight of chromosome: 2.5 x 109 Total number of base pairs: 4 x 106
- 32. E. coli statistics: WHOLE CELL: Length = 2 ; width = 0.5 . CHROMOSOME: length = 1,354 = 1.35 mm! (The chromosome is 700x as long as the entire cell!) Molecular weight of chromosome: 2.5 x 109 Total number of base pairs: 4 x 106
- 33. E. coli statistics: WHOLE CELL: Length = 2 ; width = 0.5 . CHROMOSOME: length = 1,354 = 1.35 mm! (The chromosome is 700x as long as the entire cell!) Molecular weight of chromosome: 2.5 x 109 Total number of base pairs: 4 x 106
- 34. E. coli statistics: WHOLE CELL: Length = 2 ; width = 0.5 . CHROMOSOME: length = 1,354 = 1.35 mm! (The chromosome is 700x as long as the entire cell!) Molecular weight of chromosome: 2.5 x 109 Total number of base pairs: 4 x 106
- 35. E. coli statistics: WHOLE CELL: Length = 2 ; width = 0.5 . CHROMOSOME: length = 1,354 = 1.35 mm! (The chromosome is 700x as long as the entire cell!) Molecular weight of chromosome: 2.5 x 109 Total number of base pairs: 4 x 106
- 36. HOW FAST IS THE E. COLI CHROMOSOME SPINNING? In “log phase”, E. coli replicates in 20 minutes. Under optimal conditions, the daughter cells begin to divide before the parent cells have fully separated! Arithmetic: If the E. coli chromosome has the Watson-Crick double-helical structure, then, with 4 million base pairs, there would have to be 400,000 Watson- Crick twists. Every one of these twists would have to be un-wound and re-wound in the space of 20 minutes. Conclusion: The chromosome, in log phase, MUST be spinning at 400,000/20 = 20,000 rpm!
- 37. HOW FAST IS THE E. COLI CHROMOSOME SPINNING? In “log phase”, E. coli replicates in 20 minutes. Under optimal conditions, the daughter cells begin to divide before the parent cells have fully separated! Arithmetic: If the E. coli chromosome has the Watson-Crick double-helical structure, then, with 4 million base pairs, there would have to be 400,000 Watson- Crick twists. Every one of these twists would have to be un-wound and re-wound in the space of 20 minutes. Conclusion: The chromosome, in log phase, MUST be spinning at 400,000/20 = 20,000 rpm!
- 38. HOW FAST IS THE E. COLI CHROMOSOME SPINNING? In “log phase”, E. coli replicates in 20 minutes. Under optimal conditions, the daughter cells begin to divide before the parent cells have fully separated! Arithmetic: If the E. coli chromosome has the Watson-Crick double-helical structure, then, with 4 million base pairs, there would have to be 400,000 Watson- Crick twists. Every one of these twists would have to be un-wound and re-wound in the space of 20 minutes. Conclusion: The chromosome, in log phase, MUST be spinning at 400,000/20 = 20,000 rpm!
- 39. HOW FAST IS THE E. COLI CHROMOSOME SPINNING? In “log phase”, E. coli replicates in 20 minutes. Under optimal conditions, the daughter cells begin to divide before the parent cells have fully separated! Arithmetic: If the E. coli chromosome has the Watson-Crick double-helical structure, then, with 4 million base pairs, there would have to be 400,000 Watson- Crick twists. Every one of these twists would have to be un-wound and re-wound in the space of 20 minutes. Conclusion: The chromosome, in log phase, MUST be spinning at 400,000/20 = 20,000 rpm!
- 40. “This is my Black & Decker power drill. Look what it does to this piece of wood. How fast do you think this thing is going? The manual says...1,000 rpm!” Previous slide Next slide Table Of Contents
- 41. Previous slide Next slide Table Of Contents
- 42. Is this molecule spinning at 20,000 rpm?
- 43. Are all the processes of life, including transcription, recombination and DNA repair, taking place as the chromosome spins at 20,000 rpm? Not likely! Previous slide Next slide Table Of Contents
- 44. If we attempt to evaluate Cairns’ view of circular DNA replication by examining his published writings from 1963-64, we are compelled to conclude that he did not think the problem through. How did he reach the conclusion that DNA replicated by virtue of a “swivel”? No such structure was known at the time. Why did he make no mention of the severe rotational problem involved in replicating a circular W-C structure? And, most of all, why did he not even mention in passing the seemingly-obvious possibility that DNA might be non-helical in cells? Previous slide Next slide Table Of Contents
- 45. If we attempt to evaluate Cairns’ view of circular DNA replication by examining his published writings from 1963-64, we are compelled to conclude that he did not think the problem through. How did he reach the conclusion that DNA replicated by virtue of a “swivel”? No such structure was known at the time. Why did he make no mention of the severe rotational problem involved in replicating a circular W-C structure? And, most of all, why did he not even mention in passing the seemingly-obvious possibility that DNA might be non-helical in cells? Previous slide Next slide Table Of Contents
- 46. If we attempt to evaluate Cairns’ view of circular DNA replication by examining his published writings from 1963-64, we are compelled to conclude that he did not think the problem through. How did he reach the conclusion that DNA replicated by virtue of a “swivel”? No such structure was known at the time. Why did he make no mention of the severe rotational problem involved in replicating a circular W-C structure? And, most of all, why did he not even mention in passing the seemingly-obvious possibility that DNA might be non-helical in cells?
- 47. Previous slide Next slide Table Of Contents
- 48. Picture by Mariana Ruiz & Michael Biech http://commons.wikimedia.org/wiki/File:DNA_replication_de.svg Previous slide Next slide Table Of Contents
- 49. Picture by Mariana Ruiz & Michael Biech http://commons.wikimedia.org/wiki/File:DNA_replication_de.svg Previous slide Next slide Table Of Contents
- 50. Most of the topoisomerase and gyrase research is based upon in vitro studies, where the native structure of DNA is destroyed. As soon as you remove DNA from its natural protein environment, it’s going to wind itself up into a helix, and everything you discover subsequently is at risk of being laboratory artifact. Even if these enzymes do what they are claimed to do in vitro, isn’t it possible in the living cell, where the DNA structure may be non-helical, that their true roles may be in other processes, such as DNA repair? Most of the topoisomerase and gyrase research is based upon in vitro studies, where the native structure of DNA is destroyed. As soon as you remove DNA from its natural protein environment, it’s going to wind itself up into a helix, and everything you discover subsequently is at risk of being laboratory artifact. Even if these enzymes do what they are claimed to do in vitro, isn’t it possible in the living cell, where the DNA structure may be non-helical, that their true roles may be in other processes, such as DNA repair? The typical 2-domain experiment involves either enzyme mutants, or administration of enzyme poisons, neither of which necessarily even stops DNA replication, but may merely slow it down. The products generated may or may not be supercoiled in various senses, according to 2-dimensional electrophoresis gels which are exceedingly difficult to interpret. Virtually any result reported in one lab has been contradicted by a different result elsewhere. Previous slide Next slide Table Of Contents
- 51. Besides, none of those enzymes were known in 1963. Therefore, we still have no explanation for the curious fact that Cairns insisted that DNA replication had to be by means of a “swivel”. Previous slide Next slide Table Of Contents
- 52. Three possible explanations: 1. Perhaps Cairns was not intelligent enough to figure out that DNA was not necessarily helical in cells (a very unlikely explanation). 2. Perhaps experimental evidence existed which proved, or strongly suggested, that DNA was indeed helical; not only in synthetic laboratory crystals, but in the nuclei of living cells as well. Such evidence was, in fact, starting to emerge. But there’s no evidence that Cairns, or any other speaker at Cold Spring Harbor at that time, knew about it. They certainly made no mention of it. 3. Perhaps it had already become “politically incorrect”, as early as 1963, to speak publicly of any DNA structure other than the “double helix”, because DNA had already risen to the level of a quasi-religious icon in the minds of most scientists.
- 53. Three possible explanations: 1. Perhaps Cairns was not intelligent enough to figure out that DNA was not necessarily helical in cells (a very unlikely explanation). 2. Perhaps experimental evidence existed which proved, or strongly suggested, that DNA was indeed helical; not only in synthetic laboratory crystals, but in the nuclei of living cells as well. Such evidence was, in fact, starting to emerge. But there’s no evidence that Cairns, or any other speaker at Cold Spring Harbor at that time, knew about it. They certainly made no mention of it. 3. Perhaps it had already become “politically incorrect”, as early as 1963, to speak publicly of any DNA structure other than the “double helix”, because DNA had already risen to the level of a quasi-religious icon in the minds of most scientists.
- 54. Three possible explanations: 1. Perhaps Cairns was not intelligent enough to figure out that DNA was not necessarily helical in cells (a very unlikely explanation). 2. Perhaps experimental evidence existed which proved, or strongly suggested, that DNA was indeed helical; not only in synthetic laboratory crystals, but in the nuclei of living cells as well. Such evidence was, in fact, starting to emerge. But there’s no evidence that Cairns, or any other speaker at Cold Spring Harbor at that time, knew about it. They certainly made no mention of it. 3. Perhaps it had already become “politically incorrect”, as early as 1963, to speak publicly of any DNA structure other than the “double helix”, because DNA had already risen to the level of a quasi-religious icon in the minds of most scientists.
- 55. Three possible explanations: 1. Perhaps Cairns was not intelligent enough to figure out that DNA was not necessarily helical in cells (a very unlikely explanation). 2. Perhaps experimental evidence existed which proved, or strongly suggested, that DNA was indeed helical; not only in synthetic laboratory crystals, but in the nuclei of living cells as well. Such evidence was, in fact, starting to emerge. But there’s no evidence that Cairns, or any other speaker at Cold Spring Harbor at that time, knew about it. They certainly made no mention of it. 3. Perhaps it had already become “politically incorrect”, as early as 1963, to speak publicly of any DNA structure other than the “double helix”, because DNA had already risen to the level of a quasi-religious icon in the minds of most scientists.
- 56. Three possible explanations: 1. Perhaps Cairns was not intelligent enough to figure out that DNA was not necessarily helical in cells (a very unlikely explanation). 2. Perhaps experimental evidence existed which proved, or strongly suggested, that DNA was indeed helical; not only in synthetic laboratory crystals, but in the nuclei of living cells as well. Such evidence was, in fact, starting to emerge. But there’s no evidence that Cairns, or any other speaker at Cold Spring Harbor at that time, knew about it. They certainly made no mention of it. 3. Perhaps it had already become “politically incorrect”, as early as 1963, to speak publicly of any DNA structure other than the “double helix”, because DNA had already risen to the level of a quasi-religious icon in the minds of most scientists.
- 57. Three possible explanations: 1. Perhaps Cairns was not intelligent enough to figure out that DNA was not necessarily helical in cells (a very unlikely explanation). 2. Perhaps experimental evidence existed which proved, or strongly suggested, that DNA was indeed helical; not only in synthetic laboratory crystals, but in the nuclei of living cells as well. Such evidence was, in fact, starting to emerge. But there’s no evidence that Cairns, or any other speaker at Cold Spring Harbor at that time, knew about it. They certainly made no mention of it. 3. It had already become “politically incorrect”, as early as 1963, to speak publicly of any DNA structure other than the “double helix”, because DNA had already risen to the level of a quasi-religious icon in the minds of most scientists.
- 58. “DNA is a helix.” “No other structure is possible.” “No other structure can even be discussed.”
- 59. Why on earth should DNA, in the presence of the strongly-charged basic proteins of the cell nucleus, have the same structure as DNA in the laboratory, where all those charged proteins have been artifactually removed? Is there any reason to even expect that? A Logical Question Arises: Previous slide Next slide Table Of Contents
- 60. Roses with trellis Previous slide Next slide Table Of Contents
- 61. Roses without trellis Previous slide Next slide Table Of Contents
- 62. Roses without trellis Previous slide Next slide Table Of Contents
- 63. Man with cane Previous slide Next slide Table Of Contents
- 64. Man without cane Previous slide Next slide Table Of Contents
- 65. Man without cane Previous slide Next slide Table Of Contents
- 66. Previous slide Next slide Table Of Contents
- 67. Previous slide Next slide Table Of Contents
- 68. Previous slide Next slide Table Of Contents
- 69. Previous slide Next slide Table Of Contents
- 70. Previous slide Next slide Table Of Contents
- 71. Enzyme Changing Shape Previous slide Next slide Table Of Contents
- 72. Previous slide Next slide Table Of Contents
- 74. Reasonable, always Reasonable. But always?
- 75. Introduction to alkali denaturation of circular DNA. Previous slide Next slide Table Of Contents
- 76. Circular DNA is resistant to denaturation: •The strands of circular DNA do not separate when boiled. •The strands of circular DNA do not separate at alkaline pH. Previous slide Next slide Table Of Contents
- 77. Circular DNA is resistant to denaturation: •The strands of circular DNA do not separate when boiled. •The strands of circular DNA do not separate at alkaline pH. Previous slide Next slide Table Of Contents
- 78. Circular DNA is resistant to denaturation: •The strands of circular DNA do not separate when boiled. •The strands of circular DNA do not separate at alkaline pH. Previous slide Next slide Table Of Contents
- 79. (Typical illustration of change in A260 upon denaturation; in this instance for linear DNA upon heating). Previous slide Next slide Table Of Contents
- 80. Previous slide Next slide Table Of Contents
- 81. "Is it possible that there's something about circularity which imparts unexpected characteristics on DNA, so that under conditions where the strands would separate, if linear, they do not separate when circular?" Previous slide Next slide Table Of Contents
- 82. Previous slide Next slide Table Of Contents The answer is emphatically “yes”. Circular DNA turns out to have properties entirely different than those of its linear cousins.
- 83. Previous slide Next slide Table Of Contents Robert Warner Mark Rush William Strider (Note: Data shown are for x174. Vinograd’s Polyoma data were essentially the same).
- 84. Previous slide Next slide Table Of Contents x174 “RF” (Replicative Form)
- 85. Previous slide Next slide Table Of Contents
- 86. Previous slide Next slide Table Of Contents
- 87. Previous slide Next slide Table Of Contents
- 88. Previous slide Next slide Table Of Contents
- 89. Previous slide Next slide Table Of Contents
- 90. Previous slide Next slide Table Of Contents
- 91. Previous slide Next slide Table Of Contents
- 92. Previous slide Next slide Table Of Contents
- 93. Previous slide Next slide Table Of Contents
- 94. Previous slide Next slide Table Of Contents
- 95. Previous slide Next slide Table Of Contents “Underwound”?
- 96. Helix-superhelix Previous slide Next slide Table Of Contents Secondary (helical) twists Tertiary (superhelical) twists
- 97. Helix-superhelix Previous slide Next slide Table Of Contents
- 98. Helix-superhelix Previous slide Next slide Table Of Contents
- 99. The Three Basic Principles of DNA Topology Previous slide Next slide Table Of Contents
- 100. The 1st Principle The number of secondary helical turns in fully-intact circular DNA, when it is constrained to lie flat, i.e., in a plane, is absolutely fixed at the time the rings are closed. In our so-called “heretical”, non-helical model, to be presented shortly, that number is zero. In the “classic” Watson-Crick crystal structure for linear DNA, the number is one complete right-handed helical turn per 34 Å of length. In circular DNA, as isolated in nature, if you believe in the Watson-Crick structure, then the number actually turns out to be 5-7% fewer turns than found in corresponding linear DNA, as we shall explain shortly. Previous slide Next slide Table Of Contents Whatever the number is, it cannot be changed unless one of the strands is broken open. Whatever the number is, it cannot be changed unless one of the strands is broken open.
- 101. The 2nd Principle Under physiological conditions of pH, temperature and ionic strength, the only known fully-relaxed, unstrained structure for DNA of average base composition is the Watson-Crick structure, with one full secondary helical turn per 34 Å of length. Previous slide Next slide Table Of Contents A circular duplex chromosome which, at the moment of covalent closure, has either a greater of lesser number of secondary helical turns per unit length will be under strain. A circular duplex chromosome which, at the moment of covalent closure, has either a greater of lesser number of secondary helical turns per unit length will be under strain.
- 102. Previous slide Next slide Table Of Contents
- 103. The 3rd principle Tertiary, i.e., superhelical twists, increase the tightness of the secondary winding of a helix whose twist is in the same direction, and decrease the tightness of the winding of a helix whose twist is in the opposite direction. If you don’t understand what I mean, relax, because I’m going to show you some models. This is the sort of thing which is very easy to see and grasp, but very difficult to explain in words. Previous slide Next slide Table Of Contents
- 104. 2-Twist Helix-to-Superhelix Previous slide Next slide Table Of Contents
- 105. 2-Twist Helix-to-Superhelix Previous slide Next slide Table Of Contents
- 106. 2-Twist Helix-to-Superhelix Previous slide Next slide Table Of Contents
- 107. What if the chromosome didn't "like" the way it was twisted? That is, suppose there were too many, or too few helical turns, or even left- handed turns? What could the chromosome do to "cure" the defect? Previous slide Next slide Table Of Contents
- 108. Previous slide Next slide Table Of Contents 2 RIGHT- HANDED secondary twists 2 LEFT- HANDED tertiary twists =
- 109. NOW, you may be wondering what benefit there is in removing secondary twists, if we're just going to add tertiary superhelical twists. That’s a good question. In order to see the benefit, let’s look at a real model made from rope. Previous slide Next slide Table Of Contents
- 110. Previous slide Next slide Table Of Contents
- 111. Previous slide Next slide Table Of Contents
- 112. Previous slide Next slide Table Of Contents
- 113. Previous slide Next slide Table Of Contents
- 114. Previous slide Next slide Table Of Contents
- 115. Previous slide Next slide Table Of Contents
- 116. Bearing in mind what I said about the chromosome not being “happy” when either under- or over-wound, we can now see the "secret" of reducing helical strain by the winding in (or out) of superhelical twists in the opposite sense; namely that a small number of superhelical twists in the opposite sense do in fact have the ability to significantly reduce secondary helical strain, and add no additional element of steric hindrance to the molecule. Previous slide Next slide Table Of Contents
- 117. The process of removing secondary helical strain due to over- or under-winding through the formation of superhelical twists in the opposite sense cannot, however, continue indefinitely. There does come a point where an excessive number of superhelical twists will cause the DNA strands to be brought so close together that the negatively-charged phosphate groups will begin to repel each other, discouraging further superhelical twisting. This is illustrated in the following drawing: Previous slide Next slide Table Of Contents
- 118. Previous slide Next slide Table Of Contents
- 119. SUMMARY Previous slide Next slide Table Of Contents
- 120. SUMMARY Previous slide Next slide Table Of Contents
- 121. SUMMARY Previous slide Next slide Table Of Contents
- 122. SUMMARY Previous slide Next slide Table Of Contents
- 123. SUMMARY Previous slide Next slide Table Of Contents
- 124. SUMMARY Previous slide Next slide Table Of Contents
- 125. SUMMARY Previous slide Next slide Table Of Contents
- 126. Alkali denaturation of circular DNA, according to “traditional” theory Rush, M.G. and R.C. Warner (1970). Alkali denaturation of covalently closed circular duplex deoxyribonucleic acid. J. Biol. Chem. 245, 2704-2708. Previous slide Next slide Table Of Contents
- 127. Previous slide Next slide Table Of Contents
- 128. Previous slide Next slide Table Of Contents
- 129. Previous slide Next slide Table Of Contents
- 130. I’ve always found it odd that people are so ready to believe that chromosomes are routinely underwound. Exactly why should we believe this? There’s really no logical reason for it. It’s merely another assumption the “helicists” must make in order to force the data to accommodate to the Watson-Crick helical structure. The closest thing to a rational explanation offered by the “helicists” is that the 25-30 supertwists somehow facilitate packing of the DNA in the virion — a rather arbitrary proposal; one which hardly scratches the surface of the almost-incomprehensible problem of packing huge lengths of chromosomal DNA into the tiny spaces Nature provides for them. (I have a much better explanation, but we’re not up to that yet).
- 131. Previous slide Next slide Table Of Contents
- 132. Previous slide Next slide Table Of Contents
- 133. Previous slide Next slide Table Of Contents
- 134. Previous slide Next slide Table Of Contents
- 135. Form IV (?) Previous slide Next slide Table Of Contents
- 136. What happens if a nick is introduced into a closed-circular chromosome? Previous slide Next slide Table Of Contents
- 137. Previous slide Next slide Table Of Contents
- 138. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 139. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 140. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 141. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 142. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 143. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 144. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 145. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 146. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 147. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 148. Alkali Denaturation Titration Explained in terms of the TN model (Topologically Non-linked) model Previous slide Next slide Table Of Contents TN Topologically Non-linked
- 149. Previous slide Next slide Table Of Contents
- 150. Previous slide Next slide Table Of Contents
- 151. A B C
- 152. A B C
- 153. A B C
- 159. A B C
- 160. A B C
- 161. A B C
- 162. Previous slide Next slide Table Of Contents Not realistic More realistic
- 163. Side-By-Side Structure Rodley, G.A., R.S. Scobie, R.H.T. Bates and R.M. Lewitt (1976). A possible conformation for double-stranded polynucleotides. Proc. Natl. Acad. Sci., USA. 73, 2959-2963. Previous slide Next slide Table Of Contents
- 164. Side-By-Side Structure Rodley, G.A., R.S. Scobie, R.H.T. Bates and R.M. Lewitt (1976). A possible conformation for double-stranded polynucleotides. Proc. Natl. Acad. Sci., USA. 73, 2959-2963. Previous slide Next slide Table Of Contents
- 165. Paranaemic Structure Delmonte, C. and Mann, L., 2003. Variety in DNA secondary structure. Curr. Sci. 85, 1564-1570. Assymetric structure whose dimensions accord with measurements of monolayer DNA films in Langmuir troughs. (James T.W. and Mazia, D., 1953. Biochim Biophys Acta, 10:367-370.) Assymetric structure whose dimensions accord with measurements of monolayer DNA films in Langmuir troughs. (James T.W. and Mazia, D., 1953. Biochim Biophys Acta, 10:367-370.)
- 166. Any one of these models would allow DNA to replicate without a swivel, and without the need to account for a rotational speed of 20,000 rpm at the replicative site. I actually believe that DNA, in vivo, has the structure proposed by Tai Te Wu, which is taken up in the second presentation of this series, “The Probable Structure of the Protamine-DNA Complex”. For the remainder of the present discussion, I shall not assume anything in particular about the structure of circular DNA other than that the strands are topologically non-linked (TN).
- 167. Crux of the matter. A question: Let us assume, for the sake of argument, that circular duplex DNA has the TN structure. What can we predict about the topology of the chromosome, specifically with respect to tertiary superhelical winding?
- 168. Previous slide Next slide Table Of Contents
- 169. Previous slide Next slide Table Of Contents
- 170. Previous slide Next slide Table Of Contents
- 171. Previous slide Next slide Table Of Contents
- 172. But you do need 3 things: 1. You need an IQ above 100. 2. You need sufficient humility to admit that it’s at least possible that there’s something basic about DNA that you don’t know, and, 3. You need to be sufficiently free of financial attachments to publicly-traded biotech companies to maintain the capacity for independent thought, as dramatized by the following graph, which shows what happens when scientists get too rich, and too successful: Previous slide Next slide Table Of Contents
- 173. Previous slide Next slide Table Of Contents
- 174. The Answer: If DNA had any TN structure, it would have to be isolated as a right-handed superhelix. Why?
- 175. Topologically, TN DNA is best thought of as model “A”, being half right-handed, and half left-handed. Previous slide Next slide Table Of Contents
- 176. Model “B” is topologically the same, only here the distribution of RH and LH turns is different. There is no reason to presume, however, that the energetics, with respect to superhelical tertiary winding, will be different simply because the RH and LH portions are not segregated into separate regions. Previous slide Next slide Table Of Contents
- 177. Model “A”, topologically speaking, is 50% left-handed, which cannot change unless a swivel is created by the rupturing of at least one covalent bond in the sugar-phosphate backbone. What do we know about left-handed DNA? Previous slide Next slide Table Of Contents 50% LH’d
- 178. We know that the LH helix (the “Z” form) does not normally occur in natural linear DNA of average base sequence, but only in certain synthetic copolymers. Therefore, there is no reason to doubt that DNA, under physiological conditions, will assume the Watson-Crick all-RH helical structure whenever possible. Here, however, it is not possible because the LH portion is locked in at the time of creation. We must therefore conclude that a chromosome constrained to be 50% LH, in the absence of the nuclear proteins necessary to support this structure, will be topologically unstable, and will do whatever it can to convert to a 100% RH helix. Previous slide Next slide Table Of Contents
- 179. And what can it do? The left-handed secondary turns are locked in, and cannot be removed. Only one option remains: It can take on RH tertiary superhelical turns, each of which unwinds one of the unwanted LH secondary helical turns. In the thousand-fold over-simplified drawing shown here, the bottom of the chromosome has exactly 4 LH helical turns. If such a simple structure actually existed, then the mere introduction of 4 RH superhelical turns would totally untwist the bottom into a pair of parallel lines, and 4 more would twist it in the opposite direction, yielding a secondary structure which was an all-RH Watson- Crick helix! (This principle was illustrated in the rope models above). Previous slide Next slide Table Of Contents
- 180. In practice, it will be impossible for the molecule to remove all its LH turns, because of the problem we discussed earlier, namely that of steric hindrance, which will increase as the number of superhelical turns increases. “C” depicts, schematically, the equilibrium state, beyond which there can be no further supertwisting. Previous slide Next slide Table Of Contents
- 181. The point is, that if TN DNA existed, it would have to be a RH superhelix, and the extent of right-handed superhelicity would have to the maximum extent possible, established by an equilibrium between the push of the LH secondary helical winding as it tries to unwind, but balanced by the resistance of the structure to very high degrees of superhelical twisting. Previous slide Next slide Table Of Contents The point is, that if TN DNA existed, it would have to be a RH superhelix, and the extent of right-handed superhelicity would have to the maximum extent possible, established by an equilibrium between the push of the LH secondary helical winding as it tries to unwind, but balanced by the resistance of the structure to very high degrees of superhelical twisting. The point is, that if TN DNA existed, it would have to be a RH superhelix, and the extent of right-handed superhelicity would have to the maximum extent possible, established by an equilibrium between the push of the LH secondary helical winding as it tries to unwind, but balanced by the resistance of the structure to very high degrees of superhelical twisting.
- 182. IF WHAT I JUST SAID IS NOT COMPLETELY CLEAR, THEN YOU DO NOT UNDERSTAND THE PROBLEM ENOUGH TO PROCEED. Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 183. IF WHAT I JUST SAID IS NOT COMPLETELY CLEAR, THEN YOU DO NOT UNDERSTAND THE PROBLEM ENOUGH TO PROCEED. YOU MUST, IN THAT CASE, REVIEW THE PREVIOUS SLIDES UNTIL THIS CONCEPT BECOMES 100% CLEAR. Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 184. Please note that I have not alleged to have “proven” that DNA has any structure other than the Watson-Crick structure. IF WHAT I JUST SAID IS NOT COMPLETELY CLEAR, THEN YOU DO NOT UNDERSTAND THE PROBLEM ENOUGH TO PROCEED. YOU MUST, IN THAT CASE, REVIEW THE PREVIOUS SLIDES UNTIL THIS CONCEPT BECOMES 100% CLEAR. Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 185. I have merely stated, with essentially 100% certainty, that if DNA had a TN structure, then it would have to be isolated as a right-handed superhelix, because the right-handed portion would energetically overwhelm the left-handed portion, forcing some of the left-handed turns to unwind into right-handed superhelical turns. IF WHAT I JUST SAID IS NOT COMPLETELY CLEAR, THEN YOU DO NOT UNDERSTAND THE PROBLEM ENOUGH TO PROCEED. YOU MUST, IN THAT CASE, REVIEW THE PREVIOUS SLIDES UNTIL THIS CONCEPT BECOMES 100% CLEAR. Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 186. This conclusion does not arise from research, and does not require any. It arises from a common-sense consideration of what makes sense topologically. IF WHAT I JUST SAID IS NOT COMPLETELY CLEAR, THEN YOU DO NOT UNDERSTAND THE PROBLEM ENOUGH TO PROCEED. YOU MUST, IN THAT CASE, REVIEW THE PREVIOUS SLIDES UNTIL THIS CONCEPT BECOMES 100% CLEAR. Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 187. If there was a species of hemp rope which had a natural inclination toward a right-handed twist, and you forced it into a circle which was 50% left-handed, the conclusion would be just as valid for the rope as it is for DNA. This is not chemistry. It is topology. IF WHAT I JUST SAID IS NOT COMPLETELY CLEAR, THEN YOU DO NOT UNDERSTAND THE PROBLEM ENOUGH TO PROCEED. YOU MUST, IN THAT CASE, REVIEW THE PREVIOUS SLIDES UNTIL THIS CONCEPT BECOMES 100% CLEAR. Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 188. THEREFORE, ANYONE WHO SAYS THAT: Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 189. THEREFORE, ANYONE WHO SAYS THAT: • THE WATSON-CRICK STRUCTURE IS A RIGHT-HANDED HELIX Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 190. THEREFORE, ANYONE WHO SAYS THAT: • THE WATSON-CRICK STRUCTURE IS A RIGHT-HANDED HELIX • DNA IS ISOLATED, IN NATURE, AS A RIGHT-HANDED SUPERHELIX Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 191. THEREFORE, ANYONE WHO SAYS THAT: • THE WATSON-CRICK STRUCTURE IS A RIGHT-HANDED HELIX • DNA IS ISOLATED, IN NATURE, AS A RIGHT-HANDED SUPERHELIX • “THEREFORE, ANY DNA WHICH IS A RIGHT-HANDED SUPERHELIX MUST HAVE THE WATSON-CRICK STRUCTURE”… Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 192. …DOES NOT KNOW WHAT HE’S TALKING ABOUT! Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 193. I shall now proceed with the assumption that you understand that TN DNA, if it exists, would have to be a RH superhelix at normal pH and ionic strength. Previous slide Next slide Table Of Contents (This portion of the show is silent)
- 194. Complete Explanation of the Alkali Denaturation Titration Curve in terms of the TN (Topologically Non-linked) Model Previous slide Next slide Table Of Contents
- 195. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 196. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 197. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 198. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 199. Because of steric hindrance, there’s an upper limit on the maximum number of possible superhelical turns. Previous slide Next slide Table Of Contents
- 200. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 201. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 202. The RL Conversion Previous slide Next slide Table Of Contents
- 203. 1. ORD spectrum of DNA inverts in aqueous methanol: Travers, F., A.M. Michelson and P. Douzou (1970). Conformational changes of nucleic acids in methanol-water solutions at low temperature. Biochim Biophys Acta 217, 1-6. 2. CD spectrum of DNA inverts at high salt concentration: Zimmer, C. and G. Luck (1974). Conformation and reactivity of DNA. IV. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim. Biophys. Acta 361, 11-32. 3. CD spectrum inverts in presence of mitomycin C: Mercado, C.M. and M. Tomasz (1977). Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry 16, 2040-2046. 4. X-ray crystallography of synthetic co-polymer with inverted CD spectrum reveals LH helix: Mitsui, Y., R. Langridge, B.E. Shortle, C.R. Cantor, R.C. Grant, M. Kodama, and R.D. Wells (1970). Physical and enzymatic studies on poly d(I-C).poly d(I-C), an unusual double-helical DNA. Nature 228, 1166-1169. Wang, A.H.J., G.J. Quigley, F.J. Kolpak, J.L. Crawford, J.H. Van Boom, G. Van Der Marel and A. Rich (1979). Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282, 680-686.
- 204. 1. ORD spectrum of DNA inverts in aqueous methanol: Travers, F., A.M. Michelson and P. Douzou (1970). Conformational changes of nucleic acids in methanol-water solutions at low temperature. Biochim Biophys Acta 217, 1-6. 2. CD spectrum of DNA inverts at high salt concentration: Zimmer, C. and G. Luck (1974). Conformation and reactivity of DNA. IV. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim. Biophys. Acta 361, 11-32. 3. CD spectrum inverts in presence of mitomycin C: Mercado, C.M. and M. Tomasz (1977). Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry 16, 2040-2046. 4. X-ray crystallography of synthetic co-polymer with inverted CD spectrum reveals LH helix: Mitsui, Y., R. Langridge, B.E. Shortle, C.R. Cantor, R.C. Grant, M. Kodama, and R.D. Wells (1970). Physical and enzymatic studies on poly d(I-C).poly d(I-C), an unusual double-helical DNA. Nature 228, 1166-1169. Wang, A.H.J., G.J. Quigley, F.J. Kolpak, J.L. Crawford, J.H. Van Boom, G. Van Der Marel and A. Rich (1979). Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282, 680-686.
- 205. 1. ORD spectrum of DNA inverts in aqueous methanol: Travers, F., A.M. Michelson and P. Douzou (1970). Conformational changes of nucleic acids in methanol-water solutions at low temperature. Biochim Biophys Acta 217, 1-6. 2. CD spectrum of DNA inverts at high salt concentration: Zimmer, C. and G. Luck (1974). Conformation and reactivity of DNA. IV. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim. Biophys. Acta 361, 11-32. 3. CD spectrum inverts in presence of mitomycin C: Mercado, C.M. and M. Tomasz (1977). Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry 16, 2040-2046. 4. X-ray crystallography of synthetic co-polymer with inverted CD spectrum reveals LH helix: Mitsui, Y., R. Langridge, B.E. Shortle, C.R. Cantor, R.C. Grant, M. Kodama, and R.D. Wells (1970). Physical and enzymatic studies on poly d(I-C).poly d(I-C), an unusual double-helical DNA. Nature 228, 1166-1169. Wang, A.H.J., G.J. Quigley, F.J. Kolpak, J.L. Crawford, J.H. Van Boom, G. Van Der Marel and A. Rich (1979). Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282, 680-686.
- 206. 1. ORD spectrum of DNA inverts in aqueous methanol: Travers, F., A.M. Michelson and P. Douzou (1970). Conformational changes of nucleic acids in methanol-water solutions at low temperature. Biochim Biophys Acta 217, 1-6. 2. CD spectrum of DNA inverts at high salt concentration: Zimmer, C. and G. Luck (1974). Conformation and reactivity of DNA. IV. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim. Biophys. Acta 361, 11-32. 3. CD spectrum inverts in presence of mitomycin C: Mercado, C.M. and M. Tomasz (1977). Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry 16, 2040-2046. 4. X-ray crystallography of synthetic co-polymer with inverted CD spectrum reveals LH helix: Mitsui, Y., R. Langridge, B.E. Shortle, C.R. Cantor, R.C. Grant, M. Kodama, and R.D. Wells (1970). Physical and enzymatic studies on poly d(I-C).poly d(I-C), an unusual double-helical DNA. Nature 228, 1166-1169. Wang, A.H.J., G.J. Quigley, F.J. Kolpak, J.L. Crawford, J.H. Van Boom, G. Van Der Marel and A. Rich (1979). Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282, 680-686.
- 207. Blue arrows show control DNA Orange arrows show spectral inversions
- 208. 1. ORD spectrum of DNA inverts in aqueous methanol: Travers, F., A.M. Michelson and P. Douzou (1970). Conformational changes of nucleic acids in methanol-water solutions at low temperature. Biochim Biophys Acta 217, 1-6. 2. CD spectrum of DNA inverts at high salt concentration: Zimmer, C. and G. Luck (1974). Conformation and reactivity of DNA. IV. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim. Biophys. Acta 361, 11-32. 3. CD spectrum inverts in presence of mitomycin C: Mercado, C.M. and M. Tomasz (1977). Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry 16, 2040-2046. 4. X-ray crystallography of synthetic co-polymers with inverted CD spectra has confirmed LH helix: Mitsui, Y., R. Langridge, B.E. Shortle, C.R. Cantor, R.C. Grant, M. Kodama, and R.D. Wells (1970). Physical and enzymatic studies on poly d(I-C).poly d(I-C), an unusual double-helical DNA. Nature 228, 1166-1169. Wang, A.H.J., G.J. Quigley, F.J. Kolpak, J.L. Crawford, J.H. Van Boom, G. Van Der Marel and A. Rich (1979). Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282, 680-686.
- 209. It would appear that anything which unwinds DNA might produce an RL conversion. Previous slide Next slide Table Of Contents
- 210. Z-DNA Previous slide Next slide Table Of Contents
- 211. Previous slide Next slide Table Of Contents
- 212. Length Comparison for 12 base pairs Previous slide Next slide Table Of Contents
- 213. Comparison of Z-DNA and B-DNA Rise Residues per per residue helical turn Pitch Z-DNA 3.7 Å 12 45 Å B-DNA 3.4 Å 10 34 Å LENGTH OF SEGMENT CONTAINING 12 BASE PAIRS: Z-DNA 45 Å B-DNA 41 Å Previous slide Next slide Table Of Contents No one has ever seen a Watson-Crick RH’d structure with such wide spacings, which presumably doesn’t exist. Therefore, we may be well-justified in assuming that anything which causes a 10% unwinding of DNA will precipitate an RL conversion, since only the LH’d form is known under such conditions.
- 214. Previous slide Next slide Table Of Contents
- 215. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 216. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 217. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 218. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 219. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 220. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents Why Does Form II Become Supertwisted Above pH 11.8? As the pH increases, the longitudinal base- pair spacing increases from 3.4 Å to 3.7 Å. The chromosome takes on left-handed superhelical twists. These convert the RH secondary twists, which have now become undesirable, into LH secondary twists. As the pH increases, the longitudinal base- pair spacing increases from 3.4 Å to 3.7 Å. The chromosome takes on left-handed superhelical twists. These convert the RH secondary twists, which have now become undesirable, into LH secondary twists.
- 221. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents What happens to Form II at pH 12? (That is, why do the strands separate?)
- 222. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents What happens to Form II at pH 12? The advanced degree of unwinding causes the base-pairs to reach the critical 3.7 Å spacing. This gives rise to a sudden, cooperative RL conversion. The centrifugal force of the spinning chromosome drives the strands apart. The advanced degree of unwinding causes the base-pairs to reach the critical 3.7 Å spacing. This gives rise to a sudden, cooperative RL conversion. The centrifugal force of the spinning chromosome drives the strands apart.
- 223. Previous slide Next slide Table Of Contents
- 224. Form II splits into single strands at pH 12. If the strands of Form I are Topologically Non-Linked, why don’t they also split apart at pH 12? Previous slide Next slide Table Of Contents
- 225. Two reasons: 1. Cooperative protection against early denaturation. 2. Topological properties of Form I preclude rapid RL conversion. Previous slide Next slide Table Of Contents
- 226. Two reasons: 1. Cooperative protection against early denaturation. 2. Topological properties of Form I preclude rapid RL conversion. Previous slide Next slide Table Of Contents
- 227. When DNA has no free end, twice as much energy will be required to initiate strand separation under denaturing conditions. Previous slide Next slide Table Of Contents
- 228. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents The denaturation pH of Form I, known to be about 12.3, is approximately 0.3 units higher than that of nicked Form II. Coincidence? It is therefore possible, in principle, for nicked Form II to also survive as a duplex up to pH 12.3, but it doesn’t. An RL conversion at pH 12 provides sufficient extra disruptive energy to cause strand separation at the lower pH. “Why don’t the strands of Form I also undergo a rapid change in the direction of helical winding at pH 12, and separate at that lower pH as the strands of Form II do?”
- 229. When DNA has the TN structure, every addition of a RH’d turn must be accompanied by addition of a LH’d turn, and every removal of a RH’d turn must be accompanied by removal of a LH’D turn. When DNA has the TN structure, every addition of a RH’d turn must be accompanied by addition of a LH’d turn, and every removal of a RH’d turn must be accompanied by removal of a LH’D turn. Previous slide Next slide Table Of Contents At pH 12, even if Form I “wanted” to convert to the all- LH’d form, it couldn’t, because every RH’d turn unwound would have to be accompanied by the unwinding of a LH’d turn. But in the pH range 12-12.3, LH’d DNA is not only possible, it is favored, and therefore the 50% of the chromosome which is topologically LH’d, desiring to increase, not decrease, will oppose any removal of helical turns at all.
- 230. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 231. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents
- 232. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. ? Previous slide Next slide Table Of Contents Form IV: denatured Form I.
- 233. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents Is it possible that the failure of the strands to separate at high pH is due to some unforseen structural property of Form IV, having nothing to do with topological linkage?
- 234. Structure of Form IV Previous slide Next slide Table Of Contents
- 235. Form IV (?) Previous slide Next slide Table Of Contents A “tangled mess” (?)
- 236. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents “Permanently denatured” Form IV: a vehicle for purifying viral DNA. Because of its extreme density, it would be a cinch to separate it from other proteins and nucleic acids. This, however, required that there be a way to convert the Form IV, once purified, back to native Form I.
- 237. Strider, W., M.N. Camien and R.C. Warner (1981). Renaturation of Denatured, Covalently Closed Circular DNA. J. Biol. Chem. 256, 7820-7829. Strider, W. (1971). Denatured replicative form and complex DNA of X174: Isolation, renaturation, and sedimentation properties. Ph.D. Thesis, Department of Biochemistry, New York University School of Medicine, 550 First Avenue, New York, N.Y. 10016, U.S.A. Strider, W. and R.C. Warner (1971). Denatured replicative form and complex DNA of X174: isolation and renaturation. Fed. Proc. Fed. Amer. Soc. Exp. Biol. 30(2), 1053. Renaturation of Form IV (X174 RF ) as a function of pH and temperature
- 238. Strider, W., M.N. Camien and R.C. Warner (1981). Renaturation of Denatured, Covalently Closed Circular DNA. J. Biol. Chem. 256, 7820-7829. Strider, W. (1971). Denatured replicative form and complex DNA of X174: Isolation, renaturation, and sedimentation properties. Ph.D. Thesis, Department of Biochemistry, New York University School of Medicine, 550 First Avenue, New York, N.Y. 10016, U.S.A. Strider, W. and R.C. Warner (1971). Denatured replicative form and complex DNA of X174: isolation and renaturation. Fed. Proc. Fed. Amer. Soc. Exp. Biol. 30(2), 1053. Renaturation of Form IV (X174 RF ) as a function of pH and temperature
- 239. Strider, W., M.N. Camien and R.C. Warner (1981). Renaturation of Denatured, Covalently Closed Circular DNA. J. Biol. Chem. 256, 7820-7829. Strider, W. (1971). Denatured replicative form and complex DNA of X174: Isolation, renaturation, and sedimentation properties. Ph.D. Thesis, Department of Biochemistry, New York University School of Medicine, 550 First Avenue, New York, N.Y. 10016, U.S.A. Strider, W. and R.C. Warner (1971). Denatured replicative form and complex DNA of X174: isolation and renaturation. Fed. Proc. Fed. Amer. Soc. Exp. Biol. 30(2), 1053. Renaturation of Form IV (X174 RF ) as a function of pH and temperature
- 240. Strider, W., M.N. Camien and R.C. Warner (1981). Renaturation of Denatured, Covalently Closed Circular DNA. J. Biol. Chem. 256, 7820-7829. Strider, W. (1971). Denatured replicative form and complex DNA of X174: Isolation, renaturation, and sedimentation properties. Ph.D. Thesis, Department of Biochemistry, New York University School of Medicine, 550 First Avenue, New York, N.Y. 10016, U.S.A. Strider, W. and R.C. Warner (1971). Denatured replicative form and complex DNA of X174: isolation and renaturation. Fed. Proc. Fed. Amer. Soc. Exp. Biol. 30(2), 1053. Renaturation of Form IV (X174 RF ) as a function of pH and temperature
- 241. Strider, W., M.N. Camien and R.C. Warner (1981). Renaturation of Denatured, Covalently Closed Circular DNA. J. Biol. Chem. 256, 7820-7829. Strider, W. (1971). Denatured replicative form and complex DNA of X174: Isolation, renaturation, and sedimentation properties. Ph.D. Thesis, Department of Biochemistry, New York University School of Medicine, 550 First Avenue, New York, N.Y. 10016, U.S.A. Strider, W. and R.C. Warner (1971). Denatured replicative form and complex DNA of X174: isolation and renaturation. Fed. Proc. Fed. Amer. Soc. Exp. Biol. 30(2), 1053. Renaturation of Form IV (X174 RF ) as a function of pH and temperature
- 242. Strider, W., M.N. Camien and R.C. Warner (1981). Renaturation of Denatured, Covalently Closed Circular DNA. J. Biol. Chem. 256, 7820-7829. Strider, W. (1971). Denatured replicative form and complex DNA of X174: Isolation, renaturation, and sedimentation properties. Ph.D. Thesis, Department of Biochemistry, New York University School of Medicine, 550 First Avenue, New York, N.Y. 10016, U.S.A. Strider, W. and R.C. Warner (1971). Denatured replicative form and complex DNA of X174: isolation and renaturation. Fed. Proc. Fed. Amer. Soc. Exp. Biol. 30(2), 1053. Renaturation of Form IV (X174 RF ) as a function of pH and temperature
- 243. Form IV (?) Previous slide Next slide Table Of Contents A “tangled mess” (?)
- 244. Form I: covalently- closed-circular duplex DNA. Form II: circular duplex DNA, nicked in one strand. Form III: circular duplex DNA, nicked in both strands (i.e., linear DNA. Form IV: denatured Form I. Previous slide Next slide Table Of Contents There is no enormous conformational change between pH 12.3 and 13. Other than the shoulder at , the curve just proceeds upward, in an uncomplicated, orderly, and nearly-linear fashion.
- 245. Previous slide Next slide Table Of Contents Circular DNA becomes a left- handed superhelix above pH 11.8. The explanation is the unwinding of RH'd secondary helical turns, which forces the chromosome to assume the left- handed superhelical conformation.
- 246. Previous slide Next slide Table Of Contents By pH 12.3 (labeled ), the superhelical twisting has progressed to the point that the superhelix density is the same as that of the native chromosome, only with superhelical twisting now in the opposite direction.
- 247. Previous slide Next slide Table Of Contents Proposal: As the pH increases past 12.3, the superhelical twisting simply continues to increase... …with water being progressively squeezed out of the core, culminating in a highly super- twisted, relatively anhydrous structure.
- 248. At high pH, the structure becomes, in effect, a 4-stranded structure. Previous slide Next slide Table Of Contents Structures built around base-pairing can be excluded.Linus Pauling 4-stranded helix. Phosphate groups inside, stabilized by salt bridges. Bases outside, H-bonding with water.
- 249. Pauling L. & Corey R.B. A Proposed Structure for the Nucleic Acids. P.N.A.S. 39:84-97 (1953). Previous slide Next slide Table Of Contents
- 250. Pauling L. & Corey R.B. A Proposed Structure for the Nucleic Acids. P.N.A.S. 39:84-97 (1953). Previous slide Next slide Table Of Contents
- 251. Pauling L. & Corey R.B. A Proposed Structure for the Nucleic Acids. P.N.A.S. 39:84-97 (1953). Previous slide Next slide Table Of Contents 2 1 3 4-stranded structure was easier to assemble. The perfect structure for Form IV circular DNA: Denaturation brings 4 strands of DNA into close approximation, under conditions of high pH where base-pairing is ruled out.
- 252. 1. Liu DJ & Day LA. Pf1 virus structure: Helical coat protein and DNA with paraxial phosphates. Science 265:671-674, 1994. 2. Day LA, Wiseman RL & Marzec CJ. Structure models for DNA in filamentous viruses with phosphates near the center. Nucleic Acids Res, 7:1393-1403, 1979. Two papers by Loren Day describing viruses whose DNA consists of a helical duplex with the phosphate groups in the center. (PDB accession #1PFI) Previous slide Next slide Table Of Contents
- 253. Form IV – Three views Previous slide Next slide Table Of Contents
- 254. Movie showing 4 strands of DNA with phosphate groups spaced to allow 3 Å salt bridges. Previous slide Next slide Table Of Contents
- 255. Previous slide Next slide Table Of Contents This completes our review of the alkaline titration curve for Form I native circular DNA. We have shown that these data can be very satisfactorily accounted for by the TN theory, which states that the strands of circular DNA have no topological linkage. But, even though the TN theory makes sense, and is consistent with what we know about circular DNA, that doesn't make it true. What evidence is there that this structure exists in the real world? We have shown that these data can be very satisfactorily accounted for by the TN theory, which states that the strands of circular DNA have no topological linkage. But, even though the TN theory makes sense, and is consistent with what we know about circular DNA, that doesn't make it true. What evidence is there that this structure exists in the real world?
- 256. 1. FOR THE WATSON-CRICK STRUCTURE 2. FOR THE TN STRUCTURE EXPERIMENTAL EVIDENCE: Previous slide Next slide Table Of Contents
- 257. “MOUNTAIN OF EVIDENCE”? Previous slide Next slide Table Of Contents
- 258. Previous slide Next slide Table Of Contents
- 259. Watson-Crick Structure Previous slide Next slide Table Of Contents
- 260. Previous slide Next slide Table Of Contents
- 261. 1. Sebring, E.D., T.J. Kelly Jr., M.M. Thoren & N.P Salzman (1971). Structure of replicating Simian Virus 40 deoxyribonucleic acid molecules. J. Virol. 8, 478-490. 2. Jaenisch, R.; Mayer, A. & Levine, A. (1971). Replicating SV40 molecules containing closed circular template DNA strands. Nature New Biol. 233, 72-75. 3. Crick, F.H.C., J.C. Wang and W.R. Bauer (1979). Is DNA really a double helix? J. Mol. Biol. 129, 449-461. 4. Stettler, U.H., H. Weber, T. Koller and Ch. Weissmann (1979). Preparation and characterization of form V DNA, the duplex DNA resulting from association of complementary, circular single-stranded DNA. J. Mol. Biol. 131, 21-40. Previous slide Next slide Table Of Contents
- 262. The topology equation Lk = T + W {Lk linking number} {T Twist} {W Writhe} Lk Previous slide Next slide Table Of Contents
- 263. The topology equation Lk = T + W {Lk linking number} {T Twist} {W Writhe} T W Previous slide Next slide Table Of Contents (1 full helical twist per 34 Å of length).
- 264. The topology equation Lk = T + W {Lk linking number} {T Twist} {W Writhe} Lk = T + W Right-handed supertwists, such as these, are defined as being negative in value. Previous slide Next slide Table Of Contents
- 265. Sample calculation assuming the Watson-Crick Structure Known length (for x174 RF) : 5000 base-pairs Presumed “T” value (@ 10 base-pairs/W-C twist): 500 Lk = T + W = 500 – 25 = 475 500 Lk = T + W = 500 - 25 = 475 Previous slide Next slide Table Of Contents
- 266. What if the chromosome is actually in the TN (Topologically-Nonlinked) conformation? What becomes of the Topology Equation? Previous slide Next slide Table Of Contents
- 267. Lk = T + W Old values: 475 = 500 – 25 Subtract 475: -475 -475 _______________ New values: 0 = 25 – 25 Lk T W NOTE: No attempt has been made to indicate the secondary winding in either drawing. 475 = 500 – 25 -475 -475 0 = 25 – 25 NOTE: No attempt has been made to indicate the secondary winding. Previous slide Next slide Table Of Contents
- 268. What about papers which DIRECTLY investigate the Lk of the native chromosome? Previous slide Next slide Table Of Contents
- 269. 1. Sebring, E.D., T.J. Kelly Jr., M.M. Thoren & N.P Salzman (1971). Structure of replicating Simian Virus 40 deoxyribonucleic acid molecules. J. Virol. 8, 478-490. 2. Jaenisch, R.; Mayer, A. & Levine, A. (1971). Replicating SV40 molecules containing closed circular template DNA strands. Nature New Biol. 233, 72-75. Previous slide Next slide Table Of Contents
- 270. Previous slide Next slide Table Of Contents “DNA MUST have the Watson-Crick structure” (because no other structure is possible). “Circular DNA with the W-C structure is superhelical” “Therefore anything which is superhelical must have the W-C structure”. (?) “DNA MUST have the Watson-Crick structure” (because no other structure is possible). “Circular DNA with the W-C structure is superhelical” “Therefore anything which is superhelical must have the W-C structure”. (?)
- 272. Since the conclusion reached from these electron micrographs of replicative intermediates is that DNA has the W-C helical structure simply because it's superhelical, which is logically absurd, you can perhaps now see that this sort of research proves absolutely nothing. Previous slide Next slide Table Of Contents
- 273. Reminder: TN DNA, which is 50% left-handed by topology, would undoubtedly have a goodly number of RH’d supertwists, because these unwind the unwanted LH’d secondary windings. Previous slide Next slide Table Of Contents
- 274. Crick, F.H.C., J.C. Wang and W.R. Bauer Is DNA really a double helix? J. Mol. Biol. 129:449-461, 1979. Previous slide Next slide Table Of Contents
- 275. Previous slide Next slide Table Of Contents
- 276. Previous slide Next slide Table Of Contents
- 277. Previous slide Next slide Table Of Contents
- 278. Previous slide Next slide Table Of Contents
- 279. Previous slide Next slide Table Of Contents
- 280. Previous slide Next slide Table Of Contents
- 281. Previous slide Next slide Table Of Contents
- 282. Previous slide Next slide Table Of Contents
- 283. Previous slide Next slide Table Of Contents
- 284. “No other interpretation of the bands is even remotely plausible.” …the strands of plasmids do not separate when alkali-denatured, which…proves that they are topologically-linked... …this is supposed to be the conclusion of his manuscript, but…it’s the underlying assumption… Previous slide Next slide Table Of Contents
- 285. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH. 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state. 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a helix. Previous slide Next slide Table Of Contents
- 286. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH. 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state. 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix. Previous slide Next slide Table Of Contents
- 287. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH. 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state. 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix. Previous slide Next slide Table Of Contents
- 288. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH. 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state. 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix. Previous slide Next slide Table Of Contents
- 289. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH. 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state. 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix. Previous slide Next slide Table Of Contents
- 290. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH - PRESUMPTION! 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state. 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix. Previous slide Next slide Table Of Contents
- 291. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH - PRESUMPTION! 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state - PRESUMPTION! 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix. Previous slide Next slide Table Of Contents
- 292. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH - PRESUMPTION! 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state - PRESUMPTION! 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel – TRUE, but irrelevant to native structure. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix. Previous slide Next slide Table Of Contents
- 293. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH - PRESUMPTION! 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state - PRESUMPTION! 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel – TRUE, but irrelevant to native structure. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix – NOT A CONCLUSION! Previous slide Next slide Table Of Contents
- 294. 1. The DNA of a typical 5000 base-pair plasmid is a right-handed helix, since the strands do not separate at high pH. 2. This DNA has 25 superhelical twists, and therefore must have been sealed shut in the 5% underwound state. 3. The agarose gel of the products of topoisomerase treatment reveals something like 25 bands, corresponding to 25 topoisomers, each of which evidently contains 1 less superhelical twist than the one in front of it in the gel. 4. Since this is a perfectly good explanation for the bands, we may conclude that DNA is a right-handed helix. Previous slide Next slide Table Of Contents
- 296. Previous slide Next slide Table Of Contents
- 297. Previous slide Next slide Table Of Contents
- 298. Previous slide Next slide Table Of Contents
- 299. Previous slide Next slide Table Of Contents
- 301. Stettler, U.H., H. Weber, T. Koller and C. Weissmann Preparation and characterization of form V DNA, the duplex DNA resulting from association of complementary, circular single-stranded DNA. J. Mol. Biol., 131:21-40, 1979. Previous slide Next slide Table Of Contents
- 302. Crick Proposal To prepare complementary single-stranded circular DNA (i.e., “+” and “-” strands) and re- anneal it. If DNA has the Watson-Crick structure, then reannealing of the “+” and “-” strands cannot give rise to normal duplex circular DNA (unless strands are broken and re-joined). If DNA has the Watson-Crick structure, then reannealing of the “+” and “-” strands cannot give rise to normal duplex circular DNA (unless strands are broken and re-joined). To prepare complementary single-stranded circular DNA (i.e., “+” and “-” strands) and re- anneal it. Previous slide Next slide Table Of Contents
- 303. Robert W. Chambers Acting Chairman Department of Biochemistry New York University Medical School Unpublished study, 1978 “Bullet Bob” Previous slide Next slide Table Of Contents
- 304. Charles Weissmann Former Professor, Dept. of Biochemistry New York University Medical School Co-founder, Biogen, Inc. Stettler UH, Weber H, Koller T, Weissmann C. Preparation and characterization of Form V DNA, the duplex DNA resulting from association of complementary, circular single- stranded DNA. J Mol Biol, 131:21-40, 1979. Previous slide Next slide Table Of Contents
- 305. Re-anneal Previous slide Next slide Table Of Contents
- 306. Previous slide Next slide Table Of Contents
- 307. Previous slide Next slide Table Of Contents
- 308. Previous slide Next slide Table Of Contents
- 309. Previous slide Next slide Table Of Contents
- 310. Previous slide Next slide Table Of Contents
- 311. Previous slide Next slide Table Of Contents
- 312. Previous slide Next slide Table Of Contents
- 313. The fractions containing the mixture of “+” and “–” circular strands were pooled immediately, and re- annealed under the following conditions: pH 8.5, 60, 20 minutes. The product was purified and analyzed by gel electrophoresis. Previous slide Next slide Table Of Contents
- 314. Previous slide Next slide Table Of Contents Direction of electrophoresis Normal DNA ???
- 315. pH 8.5 … 60 … 20 minutes Previous slide Next slide Table Of Contents
- 316. (Data from laboratories of R.C. Warner and W. Strider.)
- 317. Previous slide Next slide Table Of Contents
- 318. Previous slide Next slide Table Of Contents What do these data predict at pH 8.5, the pH chosen by Weissmann for his reannealing?
- 319. Previous slide Next slide Table Of Contents
- 320. pH 10.4 Previous slide Next slide Table Of Contents
- 321. pH 10.4 0% ! Previous slide Next slide Table Of Contents
- 322. Conclusion: At pH 8.5, 60, there would be NO renaturation! So what is Form V?? Previous slide Next slide Table Of Contents
- 323. “What gives Dr. Biegeleisen the right to say that re-naturation data for Form IV can be applied to re-naturation of single-stranded circles?” “What gives Dr. Biegeleisen the right to say that re-naturation data for Form IV can be applied to re-naturation of single-stranded circles?” Previous slide Next slide Table Of Contents
- 324. Form 0 Separate SS Form IV Form I Form 0 Previous slide Next slide Table Of Contents
- 325. “Form 0”; bases not properly aligned Previous slide Next slide Table Of Contents
- 326. “Form 0”; bases not properly aligned Previous slide Next slide Table Of Contents
- 327. “Form 0” with base-pairing restored Previous slide Next slide Table Of Contents
- 328. IV ? ? IV ? Previous slide Next slide Table Of Contents
- 329. DNA, boiled at the indicated pH, in 0.1 M disodium phosphate, 3 mM EDTA. Gel was stained with ethidium bromide after electrophoresis. Same experiment, except 2.4 M NaCl added to boiling medium, and ethidium bromide incorporated directly into agarose. Previous slide Next slide Table Of Contents
- 330. DNA, boiled at the indicated pH, in 0.1 M disodium phosphate, 3 mM EDTA. Gel was stained with ethidium bromide after electrophoresis. Same experiment, except 2.4 M NaCl added to boiling medium, and ethidium bromide incorporated directly into agarose. Previous slide Next slide Table Of Contents
- 331. DNA, boiled at the indicated pH, in 0.1 M disodium phosphate, 3 mM EDTA. Gel was stained with ethidium bromide after electrophoresis. Same experiment, except 2.4 M NaCl added to boiling medium, and ethidium bromide incorporated directly into agarose. Previous slide Next slide Table Of Contents
- 332. DNA, boiled at the indicated pH, in 0.1 M disodium phosphate, 3 mM EDTA. Gel was stained with ethidium bromide after electrophoresis. Same experiment, except 2.4 M NaCl added to boiling medium, and ethidium bromide incorporated directly into agarose. Previous slide Next slide Table Of Contents All the single- stranded DNA, both single- stranded circular as well as linear and Form IV, are contained in this single band.
- 333. DNA, boiled at the indicated pH, in 0.1 M disodium phosphate, 3 mM EDTA. Gel was stained with ethidium bromide after electrophoresis. Same experiment, except 2.4 M NaCl added to boiling medium, and ethidium bromide incorporated directly into agarose. Previous slide Next slide Table Of Contents
- 334. Pouwels, P.H., Knijnenburg, C.M., van Rotterdam, J., Cohen, J.A. & Jansz, H.S. Structure of the replicative form of bacteriophage X174. VI. Studies on alkali- denatured double-stranded X DNA. J. Mol. Biol. 32, 169-182 (1968). Previous slide Next slide Table Of Contents
- 335. Pouwels, P.H., Knijnenburg, C.M., van Rotterdam, J., Cohen, J.A. & Jansz, H.S. Structure of the replicative form of bacteriophage X174. VI. Studies on alkali- denatured double-stranded X DNA. J. Mol. Biol. 32, 169-182 (1968). Previous slide Next slide Table Of Contents
- 336. Pouwels, P.H., Knijnenburg, C.M., van Rotterdam, J., Cohen, J.A. & Jansz, H.S. Structure of the replicative form of bacteriophage X174. VI. Studies on alkali- denatured double-stranded X DNA. J. Mol. Biol. 32, 169-182 (1968). Previous slide Next slide Table Of Contents
- 337. Pouwels, P.H., Knijnenburg, C.M., van Rotterdam, J., Cohen, J.A. & Jansz, H.S. Structure of the replicative form of bacteriophage X174. VI. Studies on alkali- denatured double-stranded X DNA. J. Mol. Biol. 32, 169-182 (1968). Previous slide Next slide Table Of Contents
- 338. IV ? Previous slide Next slide Table Of Contents These were conditions which allowed for an atypical, non-base- paired duplex structure to form, but did not allow for the wheel-like rotation necessary for the re- establishment of proper base- pairing.
- 339. Original experiment: PG (contains both strands) “Form V” Control experiment: X174 (one strand only) ??? Original experiment: PG (contains both strands) “Form V” Control experiment: X174 (one strand only) ??? Previous slide Next slide Table Of Contents
- 340. “CONTROL” EXPERIMENT DNA Solvent pH Temp. Time Experiment: PG aqueous* 8.5 60 20m “Control”: x174 organic** 8.0 20 24h *The solvent is not entirely clear. It seems that the samples were taken directly from the 10-30% sucrose gradient containing 0.8 M NaCl, 0.2 M NaOH, 0.01 M EDTA, 0.05 M Tris, pH 13, and the pH was adjusted to 8.5 with 1 M Tris-HCl, pH 7.5. Whatever the solvent composition was, it certainly contained no organic solvents. **50% formamide, 0.05 M NaCl, 5 mM Tris-HCl Previous slide Next slide Table Of Contents
- 341. “CONTROL” EXPERIMENT DNA Solvent pH Temp. Time Experiment: PG aqueous* 8.5 60 20m “Control”: x174 organic** 8.0 20 24h *The solvent is not entirely clear. It seems that the samples were taken directly from the 10-30% sucrose gradient containing 0.8 M NaCl, 0.2 M NaOH, 0.01 M EDTA, 0.05 M Tris, pH 13, and the pH was adjusted to 8.5 with 1 M Tris-HCl, pH 7.5. Whatever the solvent composition was, it certainly contained no organic solvents. **50% formamide, 0.05 M NaCl, 5 mM Tris-HCl Previous slide Next slide Table Of Contents The data, in the form in which they’re presented, suggests that x174 virion DNA did indeed become “Form V” under the conditions of the original experiment, and that new conditions were therefore sought out, where the experimental DNA turned into Form V, and the control DNA did not.
- 342. Previous slide Next slide Table Of Contents
- 343. Previous slide Next slide Table Of Contents
- 344. Previous slide Next slide Table Of Contents
- 345. Experiments which prove that DNA is not helical in cells. Previous slide Next slide Table Of Contents
- 346. Contribution of Robert W. Chambers: Separated "+" and "-" circles can re-nature to Form I after all! Vehicle: Experiment: X174 RF (contains both strands) Control: X174 virion DNA (contains one strand only) Separated "+" and "-" circles can re-nature to Form I after all! Previous slide Next slide Table Of Contents
- 347. Chambers accepts the “Crick Challenge” — the race is on! Weissmann’s work accepted by J Mol Biol — the race is over! (Chambers retires his mixture of single-stranded circular X174 “+” and “–” strands to the refrigerator.) Chambers accepts the “Crick Challenge” — the race is on! Weissmann’s work accepted by J Mol Biol — the race is over! (Chambers retires his mixture of single-stranded circular X174 “+” and “–” strands to the refrigerator.)
- 348. After 3 months in the refrigerator… Lo and behold! Form I “magically” appears! Careful analysis establishes that this is not “Form V”, but genuine Form I, but... …where did it come from? Lo and behold! Form I “magically” appears! Careful analysis establishes that this is not “Form V”, but genuine Form I, but... Previous slide Next slide Table Of Contents
- 349. Chambers’ Experiment (unpublished) Previous slide Next slide Table Of Contents
- 350. Chambers’ Experiment (unpublished) Previous slide Next slide Table Of Contents S.S. S.S. Form I
- 351. Chambers’ Experiment (unpublished) Previous slide Next slide Table Of Contents
- 352. Chambers’ Experiment (unpublished) Previous slide Next slide Table Of Contents
- 353. Chambers’ Experiment (unpublished) Previous slide Next slide Table Of Contents He proposed that the nicked strand spontaneously sealed itself, in a reaction catalyzed by the “enzyme”, water!
- 354. THERMODYNAMIC ABSURDITY: The Second Law states that the world gets more random. In a system in which there is a net accumulation of nicked strands, how can there simultaneously be a net accumulation of spontaneously-sealed strands? It’s impossible! Plausible… plausible… impossible! In a system in which there is a net accumulation of nicked strands, how can there simultaneously be a net accumulation of spontaneously-sealed strands? Previous slide Next slide Table Of Contents
- 355. Previous slide Next slide Table Of Contents I II II I
- 356. The “kicker”: Even if nicked and re-sealed DNA were energetically equivalent, so that water really could catalyze the re-sealing of nicked strands, what would cause the DNA to become 5-7% underwound before sealing, to produce the native supertwist? This form requires energy-consuming underwinding before closure, in order to generate the superhelical twists. energetically equivalent what would cause the DNA to become 5-7% underwound before sealing, to produce the native supertwist?
- 357. At some level Chambers understands the importance of what he has observed. In accordance with this, he has, in the past, given me permission to describe his work in my publications, even though he still cannot accept the most obvious conclusion. I last spoke to him about a year ago. He’s long retired, and he no longer has his laboratory notes from this period, but he stands by his observations, to this day. Since the work is un-published, you are free to doubt or disbelieve. But the Wu study, to be described next, is published, and difficult to controvert. About the best you can do is just ignore it. That, in my opinion, would be tantamount to standing on scientific thin ice. At some level Chambers understands the importance of what he has observed. In accordance with this, he has, in the past, given me permission to describe his work in my publications, even though he still cannot accept the most obvious conclusion. I last spoke to him about a year ago. He’s long retired, and he no longer has his laboratory notes from this period, but he stands by his observations, to this day. Since the work is un-published, you are free to doubt or disbelieve. But the Wu study, to be described next, is published, and difficult to controvert. About the best you can do is just ignore it. That, in my opinion, would be tantamount to standing on scientific thin ice.
- 358. Tai Te Wu Previous slide Next slide Table Of Contents
- 359. Professor; joint appointment with Biochemistry, Molecular Biology, and Cellular Biology (College of Arts and Sciences, Northwestern University). PhD, engineering, Harvard University; MBBS, medicine, Hong Kong University Email: t-wu@nwu.edu Phone: (847) 491-7849 Previous slide Next slide Table Of Contents
- 360. Wu, T.T. Secondary structures of DNA. PNAS 63:400-405, 1969. In this paper, Tai Te Wu discussed the pitch:diameter ratio for the Watson-Crick double helix at 66% and 92% humidity, as revealed by x-ray crystallography. He stated: “At 92 percent, the surrounding of the DNA fiber resembles that inside a cell, while at 66 percent, the state of the fiber becomes completely artificial. The differences…should then provide the necessary clue for us to resolve the intricate secondary structure of DNA in vivo.” After a thorough analysis of the x-ray diffraction patterns of DNA at the two humidities, Wu concluded that “if the structure of the DNA fiber at 66 percent consists of a double helix, than at 92 per cent it must consist of two double helices”. In the Wu structure, the distance between base pairs in either double helix was twice as large (i.e., about 6.8 angstroms) as in the Watson-Crick structure, but the stacking of bases at 3.4 angstroms was preserved by mutual intercalation of the base pairs of the two duplexes. Finally, he predicted that at 100% humidity, i.e., the condition prevailing in living cells, the two mutually-intercalated duplexes would lose all vestiges of helical twist and exist as a pair of mutually-intercalated “straight ladders”, each one having an inter-base spacing of about 7 angstroms.
- 361. Wu, T.T. Secondary structures of DNA. PNAS 63:400-405, 1969. In this paper, Tai Te Wu discussed the pitch:diameter ratio for the Watson-Crick double helix at 66% and 92% humidity, as revealed by x-ray crystallography. He stated: “At 92 percent, the surrounding of the DNA fiber resembles that inside a cell, while at 66 percent, the state of the fiber becomes completely artificial. The differences…should then provide the necessary clue for us to resolve the intricate secondary structure of DNA in vivo.” After a thorough analysis of the x-ray diffraction patterns of DNA at the two humidities, Wu concluded that “if the structure of the DNA fiber at 66 percent consists of a double helix, than at 92 per cent it must consist of two double helices”. In the Wu structure, the distance between base pairs in either double helix was twice as large (i.e., about 6.8 angstroms) as in the Watson-Crick structure, but the stacking of bases at 3.4 angstroms was preserved by mutual intercalation of the base pairs of the two duplexes. Finally, he predicted that at 100% humidity, i.e., the condition prevailing in living cells, the two mutually-intercalated duplexes would lose all vestiges of helical twist and exist as a pair of mutually-intercalated “straight ladders”, each one having an inter-base spacing of about 7 angstroms.
- 362. Wu, T.T. Secondary structures of DNA. PNAS 63:400-405, 1969. In this paper, Tai Te Wu discussed the pitch:diameter ratio for the Watson-Crick double helix at 66% and 92% humidity, as revealed by x-ray crystallography. He stated: “At 92 percent, the surrounding of the DNA fiber resembles that inside a cell, while at 66 percent, the state of the fiber becomes completely artificial. The differences…should then provide the necessary clue for us to resolve the intricate secondary structure of DNA in vivo.” After a thorough analysis of the x-ray diffraction patterns of DNA at the two humidities, Wu concluded that “if the structure of the DNA fiber at 66 percent consists of a double helix, than at 92 per cent it must consist of two double helices”. In the Wu structure, the distance between base pairs in either double helix was twice as large (i.e., about 6.8 angstroms) as in the Watson-Crick structure, but the stacking of bases at 3.4 angstroms was preserved by mutual intercalation of the base pairs of the two duplexes. Finally, he predicted that at 100% humidity, i.e., the condition prevailing in living cells, the two mutually-intercalated duplexes would lose all vestiges of helical twist and exist as a pair of mutually-intercalated “straight ladders”, each one having an inter-base spacing of about 7 angstroms. He proposed that the diffraction pattern of DNA at high humidity was less suggestive of a single Watson-Crick duplex, than it was of a pair of identical duplexes, each one stretched out to twice the normal length, with their base-pairs mutually intercalated. At high humidity, DNA crystallized as a four stranded structure, consisting of two intertwined Watson-Crick-type duplexes. Although the idea was considered to be an entirely reasonable interpretation of the x-ray data… genetic considerations seemed to greatly favor a 2-stranded model. Previous slide Next slide Table Of Contents
- 363. Wu, T.T. Secondary structures of DNA. PNAS 63:400-405, 1969. In this paper, Tai Te Wu discussed the pitch:diameter ratio for the Watson-Crick double helix at 66% and 92% humidity, as revealed by x-ray crystallography. He stated: “At 92 percent, the surrounding of the DNA fiber resembles that inside a cell, while at 66 percent, the state of the fiber becomes completely artificial. The differences…should then provide the necessary clue for us to resolve the intricate secondary structure of DNA in vivo.” After a thorough analysis of the x-ray diffraction patterns of DNA at the two humidities, Wu concluded that “if the structure of the DNA fiber at 66 percent consists of a double helix, than at 92 per cent it must consist of two double helices”. In the Wu structure, the distance between base pairs in either double helix was twice as large (i.e., about 6.8 angstroms) as in the Watson-Crick structure, but the stacking of bases at 3.4 angstroms was preserved by mutual intercalation of the base pairs of the two duplexes. Finally, he predicted that at 100% humidity, i.e., the condition prevailing in living cells, the two mutually-intercalated duplexes would lose all vestiges of helical twist and exist as a pair of mutually-intercalated “straight ladders”, each one having an inter-base spacing of about 7 angstroms.
- 364. Wu, T.T. Secondary structures of DNA. PNAS 63:400-405, 1969. In this paper, Tai Te Wu discussed the pitch:diameter ratio for the Watson-Crick double helix at 66% and 92% humidity, as revealed by x-ray crystallography. He stated: “At 92 percent, the surrounding of the DNA fiber resembles that inside a cell, while at 66 percent, the state of the fiber becomes completely artificial. The differences…should then provide the necessary clue for us to resolve the intricate secondary structure of DNA in vivo.” After a thorough analysis of the x-ray diffraction patterns of DNA at the two humidities, Wu concluded that “if the structure of the DNA fiber at 66 percent consists of a double helix, than at 92 per cent it must consist of two double helices”. In the Wu structure, the distance between base pairs in either double helix was twice as large (i.e., about 6.8 angstroms) as in the Watson-Crick structure, but the stacking of bases at 3.4 angstroms was preserved by mutual intercalation of the base pairs of the two duplexes. Finally, he predicted that at 100% humidity, i.e., the condition prevailing in living cells, the two mutually-intercalated duplexes would lose all vestiges of helical twist and exist as a pair of mutually-intercalated “straight ladders”, each one having an inter-base spacing of about 7 angstroms.
- 365. Wu, T.T. Secondary structures of DNA. PNAS 63:400-405, 1969. In this paper, Tai Te Wu discussed the pitch:diameter ratio for the Watson-Crick double helix at 66% and 92% humidity, as revealed by x-ray crystallography. He stated: “At 92 percent, the surrounding of the DNA fiber resembles that inside a cell, while at 66 percent, the state of the fiber becomes completely artificial. The differences…should then provide the necessary clue for us to resolve the intricate secondary structure of DNA in vivo.” After a thorough analysis of the x-ray diffraction patterns of DNA at the two humidities, Wu concluded that “if the structure of the DNA fiber at 66 percent consists of a double helix, than at 92 per cent it must consist of two double helices”. In the Wu structure, the distance between base pairs in either double helix was twice as large (i.e., about 6.8 angstroms) as in the Watson-Crick structure, but the stacking of bases at 3.4 angstroms was preserved by mutual intercalation of the base pairs of the two duplexes. Finally, he predicted that at 100% humidity, i.e., the condition prevailing in living cells, the two mutually-intercalated duplexes would lose all vestiges of helical twist and exist as a pair of mutually-intercalated “straight ladders”, each one having an inter-base spacing of about 7 angstroms.
- 366. When a double-stranded circular chromosome becomes supertwisted, the potential for forming 4-stranded structures becomes a practical reality. 2 strands 2 strands 4 strands Previous slide Next slide Table Of Contents
- 367. Previous slide Next slide Table Of Contents
- 368. Previous slide Next slide Table Of Contents
- 369. Previous slide Next slide Table Of Contents
- 370. Previous slide Next slide Table Of Contents
- 371. Previous slide Next slide Table Of Contents
- 372. Previous slide Next slide Table Of Contents
- 373. Wu R. and T.T. Wu (1996). A novel intact circular dsDNA supercoil. Bull. Math. Biol, 58:1171-1185. Previous slide Next slide Table Of Contents
- 374. "Since the complementary DNA strands of most plasmid molecules are of about equal molecular weight and their charges are the same, it will be very difficult to separate them on agarose gel electrophoresis. However, one strand of the plasmid DNA is the sense strand and one the antisense strand. While RNA transcription is occurring, D-loops are formed, with mRNA paired with one strand of DNA. Since under agarose gel electrophoresis conditions, RNA:DNA bonds are tighter than DNA:DNA bonds (J. Casey and N. Davidson, Nucl. Acids Res. 4, 1539-1552, 1977), these RNA:DNA bonds will be maintained and promote the separation, on the gel, of the weaker DNA:DNA structure". Previous slide Next slide Table Of Contents
- 375. "Since the complementary DNA strands of most plasmid molecules are of about equal molecular weight and their charges are the same, it will be very difficult to separate them on agarose gel electrophoresis. However, one strand of the plasmid DNA is the sense strand and one the antisense strand. While RNA transcription is occurring, D-loops are formed, with mRNA paired with one strand of DNA. Since under agarose gel electrophoresis conditions, RNA:DNA bonds are tighter than DNA:DNA bonds (J. Casey and N. Davidson, Nucl. Acids Res. 4, 1539-1552, 1977), these RNA:DNA bonds will be maintained and promote the separation, on the gel, of the weaker DNA:DNA structure." Previous slide Next slide Table Of Contents
- 376. "Since the complementary DNA strands of most plasmid molecules are of about equal molecular weight and their charges are the same, it will be very difficult to separate them on agarose gel electrophoresis. However, one strand of the plasmid DNA is the sense strand and one the antisense strand. While RNA transcription is occurring, D-loops are formed, with mRNA paired with one strand of DNA. Since under agarose gel electrophoresis conditions, RNA:DNA bonds are tighter than DNA:DNA bonds (J. Casey and N. Davidson, Nucl. Acids Res. 4, 1539-1552, 1977), these RNA:DNA bonds will be maintained and promote the separation, on the gel, of the weaker DNA:DNA structure." Previous slide Next slide Table Of Contents
- 377. Previous slide Next slide Table Of Contents
- 378. D-LOOP Sense Anti-sense D-LOOP The body of the chromosome has the TN structure, which is topologically 50% left-handed, and therefore inherently unstable compared with the W-C double helix. Previous slide Next slide Table Of Contents
- 379. Previous slide Next slide Table Of Contents
- 380. Previous slide Next slide Table Of Contents
- 381. Previous slide Next slide Table Of Contents
- 382. FORMI FORMI FORMI Previous slide Next slide Table Of Contents Right lanes contain Form I experimental plasmid DNA.
- 383. LINEAR LINEAR LINEAR Previous slide Next slide Table Of Contents Middle lanes: Contain DNA linearized by cleavage with the restriction endonuclease Pst I.
- 384. MARKERS MARKERS MARKERS Previous slide Next slide Table Of Contents
- 385. Previous slide Next slide Table Of Contents
- 386. Previous slide Next slide Table Of Contents
- 387. "If D-loops change the relative electrophoretic mobility of the two strands of the chromosome, why don't the linearized chromosomes also undergo strand separation in these gels?" The TN structure is, topologically speaking, 50% left handed, and is therefore inherently unstable relative to the linearized control chromosome. The latter, once nicked, is free to assume the all-right-handed W-C structure. This is the the most stable known structure for duplex DNA, and evidently does not undergo strand dissociation in these gels. Previous slide Next slide Table Of Contents
- 388. Previous slide Next slide Table Of Contents
- 389. Previous slide Next slide Table Of Contents
- 390. Previous slide Next slide Table Of Contents
- 391. “Plasmid pHTB4 [contains] the following sequence: 5' ttcgcccagcttcgctcagct … aatatgcactgtacattcca 3' 3' aagcgggtcgaagcgagtcga … ttatacgtgacatgtaaggt 5' Two probes, (5')ttcgcccagcttcgctca(3') and (3')tacgtgacatgtaaggt(5'), were synthesized by the Northwestern University Biotechnology faculty. On Southern blotting, the former probe hybridized to the faster moving band, whereas the latter hybridized to the slower moving band of the intact pHTB4 molecules separated by agarose gel electrophoresis described above.” Previous slide Next slide Table Of Contents
- 392. Wu did not stop there. Next he repeated the entire experiment with the plasmid pUC19, with the same result, that is, two bands on electrophoresis. This time he cut out the bands, isolated the DNA, and used commercially-available primers for each strand to perform DNA sequencing. The result was that each primer initiated DNA synthesis efficiently on DNA from only one of the two bands. Wu did not stop there. Next he repeated the entire experiment with the plasmid pUC19, with the same result, that is, two bands on electrophoresis Wu did not stop there. Next he repeated the entire experiment with the plasmid pUC19, with the same result, that is, two bands on electrophoresis. This time he cut out the bands, isolated the DNA, and used commercially-available primers for each strand to perform DNA sequencing. The result was that each primer initiated DNA synthesis efficiently on DNA from only one of the two bands. Previous slide Next slide Table Of Contents
- 393. Plasmid pUC19 was purchased from New England BioLabs. Wu also purchased two primers for pUC19 DNA sequencing: #1233 AGCGGATAACAATTTCACACAGGA #1224 CGCCAGGGTTTTCCCAGTCACGAC These were also purchased from New England BioLabs. DNA molecules from slower or faster band were then sequenced using these two primers separately with Sequenase, purchased from United States Biochemicals. DNA Sequencing #1233 AGCGGATAACAATTTCACACAGGA #1224 CGCCAGGGTTTTCCCAGTCACGAC Previous slide Next slide Table Of Contents
- 394. (Wu Fig. 8) “For each primer, the four lanes are A, C, G and T. For the slower band, primer #1233 (shown in the left four lanes in Fig. 8(a)) gave much more intense signal than primer #1224 (shown in the right four lanes). On the other hand, for the faster band, the reverse was true as shown in Fig. 8(b). Thus, the slower band consisted mostly of one strand, while the faster band consisted mostly of the complementary strand. Some cross- contamination could not be avoided since the two bands were very close on the agarose gel.” Previous slide Next slide Table Of Contents
- 395. (Wu Fig. 8) “For each primer, the four lanes are A, C, G and T. For the slower band, primer #1233 (shown in the left four lanes in Fig. 8(a)) gave much more intense signal than primer #1224 (shown in the right four lanes). On the other hand, for the faster band, the reverse was true as shown in Fig. 8(b). Thus, the slower band consisted mostly of one strand, while the faster band consisted mostly of the complementary strand. Some cross- contamination could not be avoided since the two bands were very close on the agarose gel.” Previous slide Next slide Table Of Contents
- 396. (Wu Fig. 8) “For each primer, the four lanes are A, C, G and T. For the slower band, primer #1233 (shown in the left four lanes in Fig. 8(a)) gave much more intense signal than primer #1224 (shown in the right four lanes). On the other hand, for the faster band, the reverse was true as shown in Fig. 8(b). Thus, the slower band consisted mostly of one strand, while the faster band consisted mostly of the complementary strand. Some cross- contamination could not be avoided since the two bands were very close on the agarose gel.” Previous slide Next slide Table Of Contents
- 397. (Wu Fig. 8) “For each primer, the four lanes are A, C, G and T. For the slower band, primer #1233 (shown in the left four lanes in Fig. 8(a)) gave much more intense signal than primer #1224 (shown in the right four lanes). On the other hand, for the faster band, the reverse was true as shown in Fig. 8(b). Thus, the slower band consisted mostly of one strand, while the faster band consisted mostly of the complementary strand. Some cross- contamination could not be avoided since the two bands were very close on the agarose gel.” Previous slide Next slide Table Of Contents
- 398. (Wu Fig. 8) “For each primer, the four lanes are A, C, G and T. For the slower band, primer #1233 (shown in the left four lanes in Fig. 8(a)) gave much more intense signal than primer #1224 (shown in the right four lanes). On the other hand, for the faster band, the reverse was true as shown in Fig. 8(b). Thus, the slower band consisted mostly of one strand, while the faster band consisted mostly of the complementary strand. Some cross- contamination could not be avoided since the two bands were very close on the agarose gel.” Previous slide Next slide Table Of Contents
- 399. (Wu Fig. 8) “For each primer, the four lanes are A, C, G and T. For the slower band, primer #1233 (shown in the left four lanes in Fig. 8(a)) gave much more intense signal than primer #1224 (shown in the right four lanes). On the other hand, for the faster band, the reverse was true as shown in Fig. 8(b). Thus, the slower band consisted mostly of one strand, while the faster band consisted mostly of the complementary strand. Some cross- contamination could not be avoided since the two bands were very close on the agarose gel.” Previous slide Next slide Table Of Contents
- 400. (Wu Fig. 8) “For each primer, the four lanes are A, C, G and T. For the slower band, primer #1233 (shown in the left four lanes in Fig. 8(a)) gave much more intense signal than primer #1224 (shown in the right four lanes). On the other hand, for the faster band, the reverse was true as shown in Fig. 8(b). Thus, the slower band consisted mostly of one strand, while the faster band consisted mostly of the complementary strand. Some cross- contamination could not be avoided since the two bands were very close on the agarose gel.” Previous slide Next slide Table Of Contents
- 401. Conclusions Previous slide Next slide Table Of Contents
- 402. “DNA MUST have the Watson-Crick helical structure, because no other structure is possible." "Circular DNA must also have the W-C structure. Since it’s invariably superhelical, it must also be underwound." "Therefore, any DNA which is found to be superhelical must have the underwound W-C structure”. “DNA MUST have the Watson-Crick helical structure, because no other structure is possible." "Circular DNA must also have the W-C structure. Since it’s invariably superhelical, it must also be underwound." "Therefore, any DNA which is found to be superhelical must have the underwound W-C structure”. Previous slide Next slide Table Of Contents
- 403. Is all, or even part of the chromosome spinning at 20,000 rpm? Or not? What do you think? Previous slide Next slide Table Of Contents
- 404. (What makes more sense?) Previous slide Next slide Table Of Contents
- 405. Previous slide Next slide Table Of Contents
- 406. Previous slide Next slide Table Of Contents
- 407. Movie showing 4 strands of DNA with phosphate groups spaced to allow 3 Å salt bridges. Previous slide Next slide Table Of Contents
- 408. Pauling L. & Corey R.B. A Proposed Structure for the Nucleic Acids. P.N.A.S. 39:84-97 (1953). Previous slide Next slide Table Of Contents
- 409. Form IV (?) (“A tangled mess”) Previous slide Next slide Table Of Contents
- 410. Previous slide Next slide Table Of Contents
- 411. “CONTROL” EXPERIMENT DNA Solvent pH Temp. Time Experiment: PG aqueous* 8.5 60 20m “Control”: x174 organic** 8.0 20 24h *The solvent is not entirely clear. It seems that the samples were taken directly from the 10-30% sucrose gradient containing 0.8 M NaCl, 0.2 M NaOH, 0.01 M EDTA, 0.05 M Tris, pH 13, and the pH was adjusted to 8.5 with 1 M Tris-HCl, pH 7.5. Whatever the solvent composition was, it certainly contained no organic solvents. **50% formamide, 0.05 M NaCl, 5 mM Tris-HCl Previous slide Next slide Table Of Contents
- 412. Chambers’ Experiment (unpublished) Previous slide Next slide Table Of Contents
- 413. Tai Te Wu Previous slide Next slide Table Of Contents
- 414. Previous slide Next slide Table Of Contents
- 415. The End Previous slide References Table Of Contents
- 416. References Previous slide Next slide Table Of Contents
- 417. Early studies on circular bacterial/viral chromosomes Cairns, J. (1963a) The bacterial chromosome and its manner of replication as seen by autoradiography. J. Mol. Biol. 6, 208-213. Cairns, J. (1963b). The chromosome of Escherichia coli. Cold Spring Harbor Symp. Quant. Biol. 28, 43-46. Vinograd, J., J. Lebowitz, R. Radloff, R. Watson and P. Laipis (1965). The twisted circular form of polyoma viral DNA. Proc. Natl. Acad. Sci. USA. 53, 1104-1111. Vinograd, J., J. Lebowitz and R. Watson (1968). Early and late helix-coil transitions in closed circular DNA. The number of superhelical turns in Polyoma DNA. J. Mol. Biol. 33, 173-197. Rush, M.G. and R.C. Warner (1970). Alkali denaturation of covalently closed circular duplex deoxyribonucleic acid. J. Biol. Chem. 245, 2704-2708. Next page Previous page Table Of Contents
- 418. Basic topology Glaubiger, D. and J.E. Hearst (1967). Effect of superhelical structure on the secondary structure of DNA rings. Biopolymers 5, 691-696. Shure, M. and J. Vinograd (1976). The number of superhelical turns in native virion SV40 DNA and Minicol DNA determined by the band counting method. Cell 8, 215- 226. Pohl, W.F. and G.W. Roberts (1978). Topological Considerations in the Theory of Replication of DNA. J. Math. Biol. 6, 383-402. Next page Previous page Table Of Contents
- 419. RL transition; left-handed DNA (Page 1) Travers, F., A.M. Michelson and P. Douzou (1970). Conformational changes of nucleic acids in methanol-water solutions at low temperature. Biochim. Biophys. Acta 217, 1-6. Mitsui, Y., R. Langridge, B.E. Shortle, C.R. Cantor, R.C. Grant, M. Kodama, and R.D. Wells (1970). Physical and enzymatic studies on poly d(I-C).poly d(I-C), an unusual double-helical DNA. Nature 228, 1166-1169. Pohl, F.M. and T.M. Jovin (1972). Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly(dG-dC). J. Mol. Biol. 67, 375-396. Ikehara, M., S. Uesugi and J. Yano (1972). Left-handed helical polynucleotides with d-sugar phosphodiester backbones. Nature New Biol., 240, 16-17. Zimmer, C. and G. Luck (1974). Conformation and reactivity of DNA. IV. Circular dichroism studies of salt-induced conformational changes of DNAs of different base composition. Biochim. Biophys. Acta 361, 11-32. Next page Previous page Table Of Contents
- 420. RL transition; left-handed DNA (Page 2) Pohl, F.M. (1976). Polymorphism of a synthetic DNA in solution. Nature 260, 365- 366. Mercado, C.M. and M. Tomasz (1977). Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry 16, 2040-2046. Wang, A.H.J., G.J. Quigley, F.J. Kolpak, J.L. Crawford, J.H. Van Boom, G. Van Der Marel and A. Rich (1979). Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282, 680-686. Nordheim, A., M.L. Pardue, E.M. Lafer, A. Möller, B.D. Stollar and A. Rich (1981). Antibodies to left-handed Z-DNA bind to interband regions of Drosophila polytene chromosomes. Nature 294, 417-422. Next page Previous page Table Of Contents
- 421. Viruses with helical DNA whose phosphate groups are inside Day, L.A., R.L. Wiseman and C.J. Marzec (1979). Structure models for DNA in filamentous viruses with phosphates near the center. Nuc Acids Res 7(6), 1393- 1403. Liu, D.J. and L.A. Day (1994). Pf1 virus structure: helical coat protein and DNA with paraxial phosphates. Science 265, 671-674. Next page Previous page Table Of Contents
- 422. Papers which purport to prove the Watson-Crick Structure Jaenisch, R., A. Mayer and A. Levine (1971). Replicating SV40 molecules containing closed circular template DNA strands. Nature New Biol. 233, 72-75. Sebring, E.D., T.J. Kelly Jr., M.M. Thoren and N.P Salzman (1971). Structure of replicating Simian Virus 40 deoxyribonucleic acid molecules. J. Virol. 8, 478- 490. Crick, F.H.C., J.C. Wang and W.R. Bauer (1979). Is DNA really a double helix? J. Mol. Biol. 129, 449-461. Stettler, U.H., H. Weber, T. Koller and Ch. Weissmann (1979). Preparation and characterization of form V DNA, the duplex DNA resulting from association of complementary, circular single-stranded DNA. J. Mol. Biol. 131, 21-40. Next page Previous page Table Of Contents
- 423. Form IV (alkali-denatured circular DNA) (Page 1) Pauling, L. and R.B. Corey (1953). A proposed structure for the nucleic acids. Proc. Natl. Acad. Sci. USA. 39, 84-97. Pouwels, P.H., Knijnenburg, C.M., van Rotterdam, J., Cohen, J.A. & Jansz, H.S. Structure of the replicative form of bacteriophage X174. VI. Studies on alkali- denatured double-stranded X DNA. J. Mol. Biol. 32, 169-182 (1968). Pouwels, P.H., J. Van Rotterdam and J.A. Cohen (1969). Structure of the replicative form of bacteriophage X174. VII. Renaturation of denatured double- stranded X DNA. J. Mol. Biol. 40, 379-390. Rush, M.G. and R.C. Warner (1970). Alkali denaturation of covalently closed circular duplex deoxyribonucleic acid. J. Biol. Chem. 245, 2704-2708. Next page Previous page Table Of Contents
- 424. Form IV (alkali-denatured circular DNA) (Page 2) Strider, W. and R.C. Warner (1971). Denatured replicative form and complex DNA of X174: isolation and renaturation. Fed. Proc. Fed. Amer. Soc. Exp. Biol. 30(2), 1053. Strider, W. (1971). Denatured replicative form and complex DNA of X174: Isolation, renaturation, and sedimentation properties. Ph.D. Thesis, Department of Biochemistry, New York University School of Medicine, 550 First Avenue, New York, N.Y. 10016, U.S.A. Strider, W., M.N. Camien and R.C. Warner (1981). Renaturation of Denatured, Covalently Closed Circular DNA. J. Biol. Chem. 256, 7820-7829. Next page Previous page Table Of Contents
- 425. Studies which support TN structures Rodley, G.A., R.S. Scobie, R.H.T. Bates and R.M. Lewitt (1976). A possible conformation for double-stranded polynucleotides. Proc. Natl. Acad. Sci., USA. 73, 2959-2963. Wu, TT (1969). Secondary structures of DNA. PNAS 63(2):400-405 (1969). Wu R. and T.T. Wu (1996). A novel intact circular dsDNA supercoil. Bull. Math. Biol, 58(6):1171-1185. The Wu paper is based in part upon: Casey, J. and N. Davidson (1977). Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucl. Acids Res. 4, 1539-1552. Next page Previous page Table Of Contents
- 426. (End of slide presentation) Next page Previous page Table Of Contents




























































































































































































































































































































































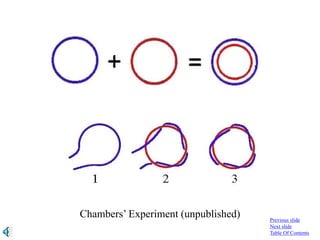









































![“Plasmid pHTB4 [contains] the following sequence:
5' ttcgcccagcttcgctcagct … aatatgcactgtacattcca 3'
3' aagcgggtcgaagcgagtcga … ttatacgtgacatgtaaggt 5'
Two probes, (5')ttcgcccagcttcgctca(3') and (3')tacgtgacatgtaaggt(5'),
were synthesized by the Northwestern University Biotechnology faculty.
On Southern blotting, the former probe hybridized to the faster moving
band, whereas the latter hybridized to the slower moving band of the intact
pHTB4 molecules separated by agarose gel electrophoresis described
above.”
Previous slide
Next slide
Table Of Contents](https://image.slidesharecdn.com/1-notahelix-slideshow-150130075949-conversion-gate02/85/1-not-a-helix-slideshow-391-320.jpg)


































