7248247.ppt
- 2. Atomic Theories • Greek Atom • Dalton Atom • Thomson Atom • Bohr Atom
- 3. The Greeks used the term atom, meaning “indivisible” [a (not) + temon (cut)] to describe the smallest part of the four substances of matter. Greek Atom The earliest recorded reference to this investigation comes from the Greeks, several hundred years bc. Scientists at that time thought that all matter was composed of four substances: earth, water, air, and fire
- 4. Dalton Atom 1) All matter is made of atoms. Atoms are indivisible and indestructible. 2) All atoms of a given element are identical in mass and properties 3) Compounds are formed by a combination of two or more different kinds of atoms. 4) A chemical reaction is a rearrangement of atoms
- 5. Thomson Atom In the late 1890s, while investigating the physical properties of cathode rays (electrons), J.J. Thomson concluded that electrons were an integral part of all atoms. He described the atom as looking something like a plum pudding, where the plums represented negative electric charges (electrons) and the pudding was a shapeless mass of uniform positive electrification. The number of electrons was thought to equal the quantity of positive electrification because the atom was known to be electrically neutral.
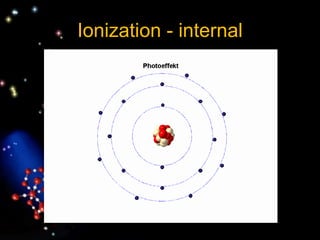
- 6. Bohr Atom In 1913, Niels Bohr improved Rutherford's description of the atom. Bohr's model was a miniature solar system in which the electrons revolved about the nucleus in prescribed orbits or energy levels. Simply put, the Bohr atom contains a small, dense, positively charged nucleus surrounded by negatively charged electrons that revolve in fixed, well-defined orbits about the nucleus. In the normal atom, the number of electrons is equal to the number of positive charges in the nucleus.
- 7. The fundamental particles of an atom are the electron, the proton, and the neutron. The atom can be viewed as a miniature solar system whose sun is the nucleus and whose planets are the electrons. The arrangement of electrons around the nucleus determines the manner in which atoms interact. Electrons are very small particles that carry one unit of negative electric charge. Their mass is only 9.1×10−31 kg. They can be pictured as revolving about the nucleus in precisely fixed orbits, just as the planets in our solar system revolve around the sun. The nucleus contains particles called nucleons, of which there are two types: protons and neutrons. Both have nearly 2000 times the mass of an electron.
- 8. The atom is mostly empty space, similar to our solar system. The nucleus of an atom is very small but contains nearly all the mass of the atom.
- 9. the atom is mostly empty space, similar to our solar system. The nucleus of an atom is very small but contains nearly all the mass of the atom. MASS Particle Location Relative Kilograms Number Charge Symbol Electron Shells 1 9.109×10−31 0 −1 − Proton Nucleus 1836 1.673×10−27 1 +1 + Neutron Nucleus 1838 1.675×10−27 1 0 O
- 10. Possible electron orbits are grouped into different “shells.” The arrangement of these shells helps reveal how an atom reacts chemically, that is, how it combines with other atoms to form molecules. Because a neutral atom has the same number of electrons in orbit as protons in the nucleus, the number of protons ultimately determines the chemical behavior of an atom. The number of protons determines the chemical element. Atoms that have the same number of protons but differ in the number of neutrons are isotopes; they behave in the same way during chemical reactions.
- 11. Electrons can exist only in certain shells, which represent different electron binding energies or energy levels. For identification purposes, electron orbital shells are given the codes K, L, M, N, and so forth, to represent the relative binding energies of electrons from closest to the nucleus to farthest from the nucleus. The closer an electron is to the nucleus, the greater is its binding energy.
- 12. In their normal state, atoms are electrically neutral; the electric charge on the atom is zero. The total number of electrons in the orbital shells is exactly equal to the number of protons in the nucleus. If an atom has an extra electron or has had an electron removed, it is said to be ionized. An ionized atom is not electrically neutral but carries a charge equal in magnitude to the difference between the numbers of electrons and protons. Atoms cannot be ionized by the addition or subtraction of protons because they are bound very strongly together, and that action would change the type of atom. An alteration in the number of neutrons does not ionize an atom because the neutron is electrically neutral.
- 16. Shell Number Shell Symbol Number of Electrons 1 K 2 2 L 8 3 M 18 4 N 32 5 O 50 6 P 72 7 Q 98 MAXIMUM ELECTRONS PER SHELL 2n2
- 17. The strength of attachment of an electron to the nucleus is called the electron binding energy, designated Eb. The closer an electron is to the nucleus, the more tightly it is bound. K-shell electrons have higher binding energies than L- shell electrons, L-shell electrons are more tightly bound to the nucleus than M- shell electrons, and so forth. Electron Binding Energy
- 18. Not all K-shell electrons of all atoms are bound with the same binding energy. The greater the total number of electrons in an atom, the more tightly each is bound.
- 19. The number of protons plus the number of neutrons in the nucleus of an atom is called the atomic mass number, symbolized by A. The atomic mass number is always a whole number. The use of atomic mass numbers is helpful in many areas of radiologic science. The chemical properties of an element are determined by the number and arrangement of electrons. In the neutral atom, the number of electrons equals the number of protons. The number of protons is called the atomic number, represented by Z.
- 20. Element Chemica l Symbol Atomic Number (Z) Atomic Mass Number (A) Number of Naturally Occurring Isotopes K-Shell Electron Binding Energy (keV) Beryllium Be 4 9 1 0.11 Carbon C 6 12 3 0.28 Oxygen O 8 16 3 0.53 Aluminum Al 13 27 1 1.56 Calcium Ca 20 40 6 4.04 Iron Fe 26 56 4 7.11 Copper Cu 29 63 2 8.98 Molybdenum Mo 42 98 7 20
- 21. Isotopes Atoms that have the same atomic number but different atomic mass numbers are isotopes.


![The Greeks used the term atom, meaning “indivisible”
[a (not) + temon (cut)] to describe the smallest part of
the four substances of matter.
Greek Atom
The earliest recorded reference to this investigation
comes from the Greeks, several hundred years bc.
Scientists at that time thought that all matter was
composed of four substances: earth, water, air, and fire](https://image.slidesharecdn.com/7248247-221011131756-dc539fc1/85/7248247-ppt-3-320.jpg)