Batteries ppt
- 1. Dr. H. S. GOUR UNIVERSITY SAGAR(M.P.) Department of physics A presentation on - Novel materials for batteries By- RAJAN KUMAR SINGH Guided by- Dr. RANVEER KUMAR
- 2. CONTENTS 1. Novel electrodes for solid state batteries (i) Introduction (ii) Requirements for electrode material designing 2. The Lithium Carbon Electrode 2.1 Graphite (i) Occurrence of Graphite (ii) Properties of Graphite (iii) Types of Graphite a: Natural Graphite b: Synthetic Graphite c: Highly Oriented Pyrolytic Graphite(HOPG) 2.2 Graphite Intercalation Compounds(GICs) (i) structure of GICs (ii) types of GICs (iii) formation of intercalation stages in GICs
- 3. CONTENTS… 3. Electrochemical intercalation of Li ion Carbon 4.Carbon – Na electrode 5. Materials for rechargeable Li batteries (i) Introduction (ii) Intercalation process (iii) Rutile type material (iv) Perovsikite type material (v) Spinel type material
- 4. 1. Novel electrodes for solid state batteries Introduction: ◙ In the recent years, a tremendous research work has developed to polymer electrolytes operating near the ambient temp. , particularily for application in solid state electrochemical devices such as:, Electro-chromic displays, sensors, super capacitors and batteries. ◙ Li polymer electrolyte batteries are used in electric vehicle development so it is huge market for EVD ◙ Electrode materials are based upon interaction compounds. ◙ Electrode materials has highly capabilities and electrode kinetics.
- 5. Requirement For Electrode Material Designing Low working potential high specific capacity good electrolyte interface
- 6. LithiumCarbonElectrode Graphite: •Most ordered carbon solid in 2-D structure. •Thermodynamically most stable form of elemental carbon. •Occurrence: Madagascar, Sri lanka, Brazil. China & India. Types of Graphite Natural graphite Synthetic graphite(Edward Goodrich) 1890 HOP G
- 7. Graphite forms in metamorphosed sedimentary rocks as an alteration of organic material. publicdomain marble metamorphosed coal (anthracite) quartzite schist gneiss by-nc-sa:bcosti by-nc-sa:RonSchott by-nc-sa:brewbooks by-nc-sa:brewbooks
- 8. ©TheodoreGray ©TheodoreGray Soft and greasy-feeling, graphite is usually found in lumps… Graphite is mined. by-nc-nd:tridymite by-nc-nd:tridymite …but occasionally in crystals.
- 10. PropertiesofGraphite Has a layered planar structure. In each layer, the carbon atoms are arranged in a honeycomb lattice with separation of 0.142nm & the distance between the plane is 0.335nm. electrically conductive. (Semi- metal) Due to weak Vander walls bond, graphite layer can be easily separated. Two form of graphite: 1. alpha(α) (hexagonal) 2. beta (β) (rhombohedral)
- 11. Applications of graphite 1. Refractory 2. Batteries 3. Steel making 4. Brake linkage 5. Pencils
- 12. Graphite Furnace Atomic Absorption Laboratory Graphite can withstand high heat. Graphite crucibles of different sizes. These crucibles can withstand temperatures up to 1500°C.
- 13. Graphite is in generators, as the carbon brushes that conduct electricity.
- 16. Lightweight sports equipment, like tennis racquets and golf clubs, are often made from graphite. Graphite Lubricant
- 17. Graphite Intercalation Compounds GICs are formed by injecting atomic/ molecular layer of other chemicals within graphite host material. Particularly physical interest due to it's high degree of order. Most important and characterization properties is, staging phenomenon. GICs 1st reported by schaffaut in 1841 but systematic study startrrd later on 1940s
- 18. Graphite Intercalation Compounds Complex material with formula CXm (as m < 1). At intercalation reaction of M species intercalating in to a host H is represented by, XM + H MxH Types of GICs Donor type GICs Acceptor type GICs Covalent & semi- covalent
- 19. 1.Doner Type GIC: @Formed with highly reducing reactive i.e. alkali & alkali earth metal. @In this GICs, the carbon layer has a negative charges e.g. Li+C- 6 2.Acceptor type GIC: @ Formed by intercalation ions or by transition metal halides or by other electron species such as Lewis acid. @ It bears opposite sign of Donner type GICs i.e. carbon have more positively charge. @ Behave as metals (synthetic metals) 3.covalent & semi-covalentGIC: Formed with highly oxidizing achives such as fluorine at high temp. or strong acids & oxanions e.g. CFX & COxHy
- 20. Structure Of Lithium - GICs LiC6 exhibit hexagonal unit cell belongs to apace group P6/mmm with parameter a=4A0 & c =3.706A0 LiC12 have a=4.288A0 & c= 7.065A0. Recently XRD study revels that presence of other superdence plane , intermediate between LiC2 & LiC6 having 8.63A0 & 11.1A0 as a and c parameter respectively.
- 21. Properties of gic Additional free carriers High in-plane mobility of graphite Higher in-plane conductivity Donor: increased off-plane conductivity Acceptor: increased off-plane resistivity Only donor GICs exhibit superconductivity
- 22. Applications of GIC Electro – chemical devices Sensors Photo – chemical devices Electro-chromic devices Superconductors Catalysts
- 23. Materials for Rechargeable Li Batteries Introduction: The science of intercalation materials deals with electrical energy storage in chemical or electrochemical form & this is the foundation for synthesizing a well defined intercalation material that can be used as, an anode and cathode in a rechargeable battery. Intercalation materials are gaining prominence in electrochemical devices, sensors & photochemical devices. Due to technical application of intercalation materials in solid state ionic devices, studied by solid state chemists, physicist & more important by material engineering & process technology.
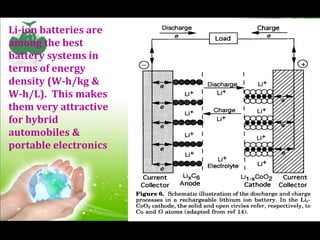
- 25. Li-ion batteries are among the best battery systems in terms of energy density (W-h/kg & W-h/L). This makes them very attractive for hybrid automobiles & portable electronics
- 26. Cathode Materials Considerations 1. The transition metal ion should have a large work function (highly oxidizing) to maximize cell voltage. 2. The cathode material should allow an insertion/extraction of a large amount of lithium to maximize the capacity. High cell capacity + high cell voltage = high energy density 3. The lithium insertion/extraction process should be reversible and should induce little or no structural changes. This prolongs the lifetime of the electrode. 4. The cathode material should have good electronic and Li+ ionic conductivities. This enhances the speed with which the battery can be discharged. 5. The cathode should be chemically stable over the entire voltage range and not react with the electrolyte. 6. The cathode material should be inexpensive, environmentally friendly and lightweight.
- 27. SPINEL TYPE MATERIALS Li1-xMn2O4 Structure type is defect spinel Mn ions occupy the octahedral sites, while Li+ resides on the tetrahedral sites. Rather poor electrical conductivity Lithium de-intercalation varies from 0 x 1, comparable to Li1-xCoO2 Presence of Mn3+ gives a Jahn-Teller distortion that limits cycling. High Li content stabilizes layer like structure. Capacity ~ 36 A-h/kg Voltage ~ 3.8 Volts Energy density ~ 137 W-h/kg Mn is cheap and non-toxic.
- 28. PEROVSKITE STRUCTURE Ba2In2O5 The brownmillerite structure can be derived from perovskite, by removing 1/6 of the oxygens and ordering the vacancies so that 50% of the smaller cations are in distorted tetrahedral coordination. In Ba2In2O5 at 800 ºC the oxygen vacancies disorder throughout the tetrahedral layer, and the ionic conductivity jumps from 10-3 S/cm to 10-1 S/cm. BaZrO3-Ba2In2O5 solid solutions absorb water to fill oxygen vacancies and become good proton conductors over the temperature range 300-700 ºC.
- 29. SOME OTHER PEROVSKITECathode (Air Electrode) (La1-xCax)MnO3 (Perovskite) (La1-xSrx)(Co1-xFex)O3 (Perovskite) (Sm1-xSrx)CoO3 (Perovskite) (Pr1-xSrx)(Co1-xMnx)O3 (Perovskite) Anode (H2/CO Electrode) Ni/Zr1-xYxO2 Composites Electrolyte (Air Electrode) Zr1-xYxO2 (Fluorite) Ce1-xRxO2 , R = Rare Earth Ion (Fluorite) Bi2-xRxO3 , R = Rare Earth Ion (Defect Fluorite) Gd1.9Ca0.1Ti2O6.95 (Pyrochlore) (La,Nd)0.8Sr0.2Ga0.8Mg0.2O2.8 (Perovskite) Interconnect (between Cathode and Anode) La1-xSrxCrO3 (Perovskite)
Editor's Notes
- #8: University of Minnesota, Department of Geology and Geophysics, Dr. Kent Kirkby. Funded in part by FIPSE. www.geo.umn.edu
- #9: University of Minnesota, Department of Geology and Geophysics, Dr. Kent Kirkby. Funded in part by FIPSE. www.geo.umn.edu
- #13: University of Minnesota, Department of Geology and Geophysics, Dr. Kent Kirkby. Funded in part by FIPSE. www.geo.umn.edu
- #14: University of Minnesota, Department of Geology and Geophysics, Dr. Kent Kirkby. Funded in part by FIPSE. www.geo.umn.edu
- #15: University of Minnesota, Department of Geology and Geophysics, Dr. Kent Kirkby. Funded in part by FIPSE. www.geo.umn.edu
- #17: University of Minnesota, Department of Geology and Geophysics, Dr. Kent Kirkby. Funded in part by FIPSE. www.geo.umn.edu