Biogeochemical cycle
- 1. Department of Fisheries and Marine Science Noakhali Science and Technology University Biogeochemical cycles
- 2. What are biogeochemical cycles? • Earth system has four parts – Atmosphere – Hydrosphere – Lithosphere – Biosphere • Biogeochemical cycles: The chemical interactions (cycles) that exist between the atmosphere, hydrosphere, lithosphere, and biosphere. • Abiotic (physio-chemical) and biotic processes drive these cycles • Focus on carbon and water cycles (but could include all necessary elements for life). N - cycle weakly touched on!
- 3. The biogeochemical cycle involves the movement of elements and compounds among the land (lithosphere), organisms, air (atmosphere) and the oceans (hydrosphere). Human activities can affect these cycles Lithosphere Atmosphere Biosphere Hydrosphere 3 The biogeochemical cycle is the continuous flow of elements and compounds between organisms and the earth The ocean plays a role in the biogeochemical cycle for elements including carbon and nitrogen As part of the carbon cycle, carbon dissolves into the surface ocean from the atmosphere and is used for photosynthesis
- 4. How do elements move through the biogeochemical cycle? Organisms use elements as nutrients and put nutrients back into the environment Elements travel among air, land and sea through physical processes 4
- 5. Various biogeochemical cycles • Water cycle • The Nitrogen Cycle • The Carbon Cycle • The Sulfur Cycle • The Phosphorus Cycle • The Hydrogen Cycle • The Oxygen Cycle
- 6. What is common amongst them? • Each compound (water, carbon, nitrogen) typically exists in all four parts of the Earth System • Transformations – are important – can lead to positive & negative consequences
- 8. Water • 3 states – Solid – Liquid – Gas • The 3 states of water are determined mostly by temperature. • Even though water is constantly changing states, the total amount of water on Earth remains constant. • Over 70% of the Earth’s surface is covered by oceans • Water is constantly being cycled between the atmosphere (air), hydrosphere (water), and lithosphere (land).
- 9. •The repeating change of water on the Earth creates a cycle • As water goes through its cycle, it can be a solid (ice), a liquid (water), or a gas (water vapor) •Ice can change to become water or water vapor •Water can change to become ice or water vapor •Water vapor can change to become ice or water.
- 10. Evaporation • The sun (temperature) is the energy force that powers the water cycle • It heats oceans, lakes, rivers and causes water to change from the liquid state to the gaseous state • The oceans contribute to about 80-90% of the water vapor in the atmosphere. • During evaporation, the impurities (for example, Salt) are left behind. – This is important because about 97% of the water on Earth is salt water (oceans) and only 3% is freshwater (rivers, streams, lakes, ponds, and in the ground).
- 11. Condensation • When atmospheric temperature decreases, the water vapor (gas) changes back into a liquid. • Condensation is the opposite of evaporation. • Small water droplets are formed in the atmosphere. • Collections of water droplets form clouds in the sky or fog at ground level. • You can see condensation on drinks in the summertime or leaves in the morning.
- 12. Water vapor condensing on a cold window.
- 13. Water vapor condensing on a cold mirror. More condensation
- 14. Water vapor condensing on the cold car windshield. More condensation
- 15. Water vapor condensing on grass or a spider web--dew. More condensation
- 16. Precipitation • Tiny water droplets bounce around in a cloud and as they hit each other, they stick together and become larger. • The clouds get heavy and eventually water falls back to the Earth. • Precipitation can occur in the form of rain, freezing rain, sleet, snow, or hail. • Most precipitation falls back into the oceans or onto land. If precipitation falls in the form of snow it can accumulate in the form of ice caps or glaciers. • Most of the condensed water in clouds does not actually fall as precipitation.
- 18. Surface Runoff • About 1/3 of the water that returns to the Earth as precipitation runs off the surface of the land, down hill, into streams, rivers, lakes, and oceans. • The other 2/3 of precipitation is evaporated, transpired, or is infiltrated into ground water. • Surface Runoff is a very important part of the water cycle because it returns water once again to the bodies of water, where evaporation occurs. • For example, when snow melts
- 20. Surface run-off from a farm field During a heavy rain, water flows over the soil filling creeks which then flow into streams and finally into rivers. The surface runoff that flows into a creek is beginning its journey back to the ocean.
- 21. Infiltration • Not all surface runoff water flows back into streams, rivers, lakes, and oceans. Some of it soaks into the ground. • Infiltration is the downward movement of water from the land surface into soil or underlying rock layers. • This water can replenish aquifers, which store large amounts of freshwater that can be removed from the ground using a water well. • Some infiltration stays close to the land surface and can seep back into surface-water bodies (and the ocean) as groundwater discharge. • Some groundwater finds openings in the land surface and comes out as freshwater springs.
- 22. Transpiration • Water is returned to the atmosphere by plants. • Water is absorbed by plants (usually through the roots) from water that is in the soil. • The water travels up through the plant and then is evaporated back into the atmosphere from the plant surface (usually the leaves).
- 23. Sublimation • Sublimation is the conversion between the solid and gaseous form of water, with no intermediate liquid stage. • This occurs when there is low atmospheric pressure. • An example of this is when snow and ice change into water vapor in the air without first melting into water. Phase Diagram for Water
- 26. • Humans use water for drinking, respiration, perspiration, and elimination of wastes are all part of this cycle • Large amounts of water are needed for most economic activities: agriculture and mining, food processing, manufacturing • Lakes and rivers provide towns and cities with a means of discharging wastes • Generation of electricity from thermal power plants • Waterways provide transportation • Recreational activities • Some people view the rivers and large lakes of this country as a part of their own identity
- 28. The Carbon Cycle Most of you is water. But a surprising part of the rest of you is carbon! This is you, without water - a lump of Carbon
- 29. What’s so Special About Carbon? • Carbon is one of the most important elements in the earth system. • The carbon atom has four valence electrons and has the ability to form bonds with as many as four other atoms including other carbon atoms. C e- e- e- e- e- e-
- 30. What’s so Special About Carbon? • Carbon can readily bond with almost any element on the periodic table. • Carbon is unparalleled among elements in its ability to bond with itself almost indefinitely, forming carbon chains, loops and braches. • What's more, the bonds forged between carbon atoms are very, very strong.
- 31. Why is Carbon so Important? All life, from a whale to a redwood tree, down to a lady bug, to an amoeba, down to our cells, even to the components inside our cells — all of it contains carbon. Carbon is the “duct tape of life,” It holds us together. Carbon is the main source of food energy. When you eat carbon molecules (plants and animals), the digestive juices in your stomach break the carbon bonds inside and release the energy in the form of calories.
- 32. Why is Carbon so Important? Hydrocarbon Molecules (coal oil, natural gas) are the primary sources of energy in our modern society. Carbon Molecules (CO2 and CH4) in the atmosphere are greenhouse gasses and are play a key role in climate change.
- 33. The Carbon Cycle • Carbon atoms continually move through living organisms, the oceans, the atmosphere, and the rocks that make up the earth system. This movement is known as the carbon cycle. • The paths taken by carbon atoms through this cycle are extremely complex, and may take years to millions of years to come full circle. • In the cycle there are various sinks, or stores, of carbon and fluxes, or processes, by which the various sinks exchange carbon on various time scales.
- 34. Dissolution Plants Phytoplankton “Biomass” Fossil Fuels The Carbon Cycle Decomposition Respiration Combustion Carbon In Atmosphere Carbon In Rocks Carbon In Ocean Water Weathering Tectonics Evaporation Lithification Soil Marine Sediment “Organic Matter” Photosynthesis Boxes are carbon sinks Arrows are carbon fluxes Consumption
- 35. Atmospheric Carbon Dioxide Plants use carbon dioxide to make their food (photosynthesis green plants are eaten by animals respiration dead remains of plants and animals decay by fungi and bacteria
- 37. Step 1: PHOTOSYNTHESIS • During photosynthesis, plants, algae, and cyanobacteria remove Carbon dioxide from the air and fix, or incorporate it into complex organic compounds such as glucose. • Photosynthesis incorporates carbon from the abiotic into the biological compounds of producers.
- 38. Step 2: DECOMPOSITION, ANIMAL & PLANT RESPIRATION, SOIL MICROORGANISM RESPIRATION. • Many of the compounds are used as fuel for cellular respiration by the producer that made them, by a consumer that eats producer, or by a decomposer that breaks down the remains of the producer or consumer. • The process of a cellular respiration returns Carbon dioxide to the atmosphere. A similar carbon cycle occurs in aquatic ecosystems between aquatic organisms and dissolved Carbon dioxide in water.
- 39. Step 3: PARTLY DECOMPOSED PLANT REMAINS (COAL) Millions of years ago vast coal beds formed from the bodies of ancient trees that were buried and subjected to anaerobic conditions before they had fully decayed.
- 40. Step 4: MARINE PLANKTON REMAINS • The oils of unicellular marine organisms probably gave rise to the underground deposits of oil and natural gas that accumulated in the geologic past. • Coal, oil, and natural gas, called fossil fuels because they formed from the remains of ancient organisms. Fossil fuels are non- renewable resources. The Earth has a finite or limited supply of these resources.
- 41. Step 5: COMBUSTION (HUMAN & NATURAL) The process of burning or combustion, may return the carbon in oil, coal, natural gas, and wood to the atmosphere. In combustion, organic molecules are rapidly oxidized (combined with oxygen) and converted carbon dioxide and water with an accompanying release of light and heat.
- 42. Step 6: BURIAL AND COMPACTION TO FORM ROCK (LIMESTONE) An even greater amount of carbon that is stored for millions of years is incorporated into the shells of marine organisms. When these organisms die, their shells sink to the ocean floor and sediments cover them forming cemented together to form limestone, a meter thick.
- 43. Step 7: EROSION OF LIMESTONE TO FORM DISSOLVED CO2 When the process of geologic uplift expose limestone, chemical and physical weathering processes slowly erode it away. This returns carbon to the water and atmosphere where it is available to participate in the carbon cycle once again.
- 44. Changes in Atmospheric C02 Dr. Pieter Tans, NOAA/ESRL (www.esrl.noaa.gov/gmd/cgg/trends)
- 45. Nitrogen Cycle
- 46. Forms of Nitrogen • Urea CO(NH2)2 • Ammonia NH3 (gaseous) • Ammonium NH4 • Nitrate NO3 • Nitrite NO2 • Atmospheric nitrogen N2 • Organic N
- 47. Nitrogen Cycle: Key Points • Nitrogen is in the atmosphere as N2 (78%) • N2 is an inert gas and cannot be used by plants or animals • N2 can be converted to a usable form via – Lightening – N-fixing plants and cyanobacteria – Industrial process (energy intensive) • Nitrogen limits plant growth • Nitrogen is easily lost from biological systems
- 48. Roles of Nitrogen • Plants and bacteria use nitrogen in the form of NH4 + or NO3 - • It serves as an electron acceptor in anaerobic environment • Nitrogen is often the most limiting nutrient in soil and water.
- 49. There are 4 phases in the cycle: • Nitrogen fixation = NH3/NH4 + • Decay = decomposing organic nitrogen into NH4 + • Nitrification = converting NH4 + to NO2 to NO3 • Denitrification = converting NO3 into N2 Micro-organisms play an important part in this cycle!
- 50. Nitrogen Fixation • The enormous energy of lightning breaks nitrogen molecules apart and enables the nitrogen atoms to combine with oxygen forming nitrogen oxides (N2O) • Nitrogen oxides dissolve in rain, forming nitrates (NO3) • Nitrates (NO3) are carried to the ground with the rain. N N O (NO3) (N2O)
- 51. Decay • Animals acquire their amino acids when they eat plants. • When animals and plants die their remains are used as food by micro-organisms such as bacteria and fungi. • Decomposers convert the nitrogen back into ammonia (NH3) Ammonia (NH3) is stored in soil. Decomposers convert organic nitrogen to ammonia (NH3) Ammonia (NH3) is used by some plants
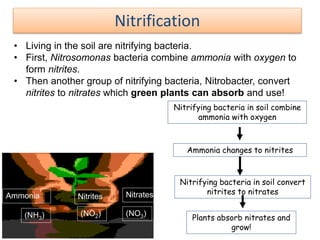
- 52. Nitrifying bacteria in soil combine ammonia with oxygen Ammonia changes to nitrites Nitrifying bacteria in soil convert nitrites to nitrates Plants absorb nitrates and grow! Ammonia Nitrites Nitrates (NH3) (NO3)(NO2) Nitrification • Living in the soil are nitrifying bacteria. • First, Nitrosomonas bacteria combine ammonia with oxygen to form nitrites. • Then another group of nitrifying bacteria, Nitrobacter, convert nitrites to nitrates which green plants can absorb and use!
- 53. Dinitrification • Removes a limiting nutrient from the environment • 4NO3 - + C6H12O6 2N2 + 6 H20 • Inhibited by O2 • Not inhibited by ammonia • Microbial reaction • Nitrate is the terminal electron acceptor
- 54. Denitrifying bacteria Nitrates (NO3 –) Detritivores Ammonium (NH4 +) Amino acids and proteins in plants and animals Detritus Assimilation by plants Nitrogen-fixing bacteria in soil Nitrogen fixation Decomposition Nitrogen-fixing bacteria in root nodules of legumes Nitrogen fixation Nitrogen (N2) in atmosphere
- 55. Oxygen Cycle
- 56. Oxygen • Oxygen – a colorless, odorless, tasteless gas • Denser than air • Poor conductor of heat and electricity • Oxygen, one of the main components of the Earth’s atmosphere, can always be found with other elements. • Two oxygen atoms make up one oxygen molecule, and three oxygen atoms together make up the molecule called ozone.
- 57. Biological Importance of Oxygen • Humans need it to breathe • Needed for decomposition of organic waste • Water can dissolve oxygen and it is this dissolved oxygen that supports aquatic life.
- 58. Ecological Importance of Oxygen • Without oxygen at the bottom of the water body, anaerobic bacteria (those that live without oxygen) produce acids. These acids not only increase acidity, but also cause a massive release of phosphorus and nitrogen, two major fertilizers, from the organic sediment and into the water column. • These same anaerobic bacteria put toxic gases in the water including hydrogen sulfide (that rotten egg smell), ammonia, carbon dioxide and methane. These gases are all toxic to fish, beneficial bacteria and insects. • Lack of bottom oxygen is the cause of odors produced by anaerobic bacteria.
- 59. The Main Reservoirs • Biosphere (living things) • Lithosphere (Earth’s crust) • Atmosphere (air) • Hydrosphere(water) The reservoirs are the locations in which oxygen is found.
- 61. What is the Oxygen Cycle? • In the oxygen cycle, oxygen atoms present in the earth circulate through a series of intricate processes. • Like the nitrogen, carbon, and water cycles, the oxygen cycle is a biogeochemical cycle. • A biogeochemical cycle is the movement of matter through the biotic and the abiotic spheres of the ecosystem.
- 62. Step One of Oxygen Cycle Plant release oxygen into the atmosphere as a by-product of photosynthesis. oxygen
- 63. Photosynthesis • Plants take in carbon dioxide and water and use them to make food. Their food is simple sugar — glucose.
- 64. Photosynthesis •Definition- process in which green plants use the energy from the sun to make carbohydrates from carbon dioxide and water in the presence of chlorophyll.
- 65. Photosynthesis (continued) • Plants pull the carbon off CO2 and use the carbon in glucose. (They do not need the oxygen for this. They get that from water, H2O.) • Plants release the oxygen (O2) back into the atmosphere. • Other organisms use the free oxygen for respiration.
- 67. Step Two of Oxygen Cycle • Animals take in oxygen through the process of respiration. • Animals then break down sugars and food.
- 68. Respiration • Process by which an organism exchanges gases with its environment • Process → oxygen is abstracted from air, transported to cells for the oxidation of organic molecules while CO2 and H2O, the products of oxidation, are returned to the environment
- 69. Step Three in Oxygen Cycle • Carbon dioxide is released by animals and used in plants in photosynthesis. • Oxygen is balanced between the atmosphere and the ocean.
- 70. How do plants contribute? • The oxygen cycle begins with plants and photosynthesis. • Through photosynthesis, plants convert the energy from the sun and water into carbohydrates and oxygen. • During the day: plants convert carbon dioxide into oxygen. • During the night: plants convert oxygen into carbon dioxide to maintain their metabolism.
- 71. How do animals contribute? • Humans and animals breathe in oxygen and breathe out carbon dioxide through their processes of metabolism, sparking the process of photosynthesis, once again linking back to the plants’ contribution to the oxygen cycle.