Carcinoma Ovary- maintenance therapy.pptx
- 2. OBJECTIVE ● WHY NEED OF MAINTENANCE THERAPY? ● EVOLUTION OF MAINTENANCE THERAPY:GENERAL OVERVIEW CHEMOTHERAPY TARGETED THERAPY PARP INHIBITORS THERAPIES CURRENTLY UNDER INVESTIGATION ● DOSAGE OF MAINTENANCE THERAPY ● DURATION OF MAINTENACE THERAPY ● SIDE EFFECT PROFILE COMPARSION
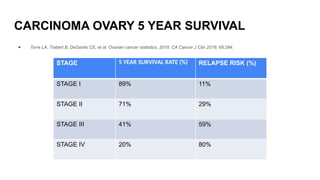
- 3. CARCINOMA OVARY 5 YEAR SURVIVAL ● Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018; 68:284. STAGE 5 YEAR SURVIVAL RATE (%) RELAPSE RISK (%) STAGE I 89% 11% STAGE II 71% 29% STAGE III 41% 59% STAGE IV 20% 80%
- 4. WHY WE NEED MAINTENANCE THERAPY? ● Debulking surgery and six courses of platinum based chemotherapy results in ‐ complete clinical remission (CCR) in up to 75% of cases(1). ● 75% of the responders will relapse within a median time of 18 to 28 months(1). ● The aim of maintenance therapy is to prolong the duration of remission and improve the overall length of survival. 1- Chen H, Fang F, Liu GJ, Xie HY, Zou J, Feng D. Maintenance chemotherapy for ovarian cancer. Cochrane Database of Systematic Reviews. 2013;1:1–40
- 5. IDEAL DRUG FOR MAINTENANCE THERAPY ● PROVEN EFFICACY ● ORAL OVER INTRAVENOUS OR OTHER ROUTES ● LESS TOXICITY WITH GREATER THERAPEUTIC BENEFIT
- 6. EVOLUTION OF MAINTENANCE THERAPY:GENERAL OVERVIEW ● Different types of chemotherapy (e.g. platinum agents, doxorubicin, topotecan or paclitaxel) were used in maintenace therapy but did not show any improvement in progression free survival (PFS) or overall survival (OS) ‐ (1). ● According to Markman 2003, 12 courses of paclitaxel can significantly prolong PFS rather than three courses. Trial was closed prematurely(2). ● An important consideration for women with advanced disease is the balance between the benefit of treatment and the harms or adverse effects that these treatments may cause(1). 1. Weekly paclitaxel- grade 2 or worse peripheral neuropathy, infection or fever, and dermatologic events 2. Intraperitoneal cisplatin- bowel obstruction. 3. Epidoxorubicin- neutropenia, anemia and thrombocytopenia. 4. Topotecan- Grade 3 to 4 neutropenia, grade 3 thrombocytopenia, nausea and vomiting. (1)Chen H, Fang F, Liu GJ, Xie HY, Zou J, Feng D. Maintenance chemotherapy for ovarian cancer. Cochrane Database of Systematic Reviews. 2013;1:1–40 (2)Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, Jiang C, Alberts D; Southwest Oncology Group; Gynecologic Oncology Group. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. J Clin Oncol. 2003 Jul 1;21(13):2460-5.
- 7. FROM CHEMOTHERAPY>>>>>TO TARGETED THERAPY ● Continued exposure to chemotherapy was associated with 1. Cumulative toxicity 2. Potential to impact later lines of therapy ● The recent development of targeted molecular therapies has resulted in great maintenance therapy options with less toxicity and greater therapeutic benefit. 1. Angiogenesis Inhibitor - Bevacizumab 2. BRCA1/2 Mutation and PARP Inhibitors 3. Therapies Currently Under Investigation
- 8. Angiogenesis Inhibitor - Bevacizumab ● Bevacizumab (Avastin) is a monoclonal antibody against vascular endothelial growth factor (VEGF). ● In 2014- Bevacizumab was approved in combination with chemotherapy in platinum- resistant recurrent ovarian cancer - Aurelia study- Improved PFS (from 3.4 to 6.7 months and ORR (11.8% versus 27.3%)(1) (1)Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G. et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–8.
- 9. From platinum resistant to platinum sensitive recurrent cancer ● In 2016 bevacizumab was approved platinum-sensitive recurrent epithelial ovarian cancer. 1. OCEANS trial- ● Bevacizumab in combination with carboplatin and paclitaxel or in combination with carboplatin and gemcitabine chemotherapy, followed by maintenance with single-agent bevacizumab ● PFS was increased from 8.4 to 12.4 months (HR=0.484 p=<0.0001), and ORR increased from 57.4% to 78.5%.(1) 2. GOG-0213 trial- (NCT00434642) ● Standard chemotherapy group(paclitaxel or carboplatin) VS the same chemotherapy regimen plus bevacizumab ● Improved median overall survival (from 37.3 months to 42.2 months HR=0.823 p=0.0447) and progression free survival (from 10.4 months to 13.8 months HR=0.628 p=0.0001) with the addition of bevacizumab ● Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2015 Oct;139(1):10-6.
- 10. From platinum sensitive recurrent cancer >>>> to maintenance setting ● In 2018 ● GOG-0218 (NCT00262847) ● Stage III or IV epithelial ovarian cancer ● Surgical debulking followed by carboplatin and paclitaxel ● Bevacizumab on cycles 2-6 (consolidation) vs cycles 2-22 (consolidation/maintenance) vs placebo ● Progression free survival was longer in the patients receiving bevacizumab at consolidation and maintenance. ● (14.1 vs. 11.2 vs. 10.3 months for consolidation/maintenance vs consolidation vs placebo HR=0.908; p=0.16 and HR=0.717; p=<0.001)) ● Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H. et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390.
- 11. International Collaboration on Ovarian Neoplasms (ICON7) trial- ● Chemotherapy alone (carboplatin and paclitaxel) VS chemotherapy with concurrent bevacizumab, followed by 12 cycles of maintenance bevacizumab ● Dose- 7.5 mg/kg every 21 days ● PFS at 36 months was 20.3 months for standard chemotherapy compared with 21.8 months for those who also received bevacizumab (p = 0.04). ● This difference was greater in patients at a high risk of progression, defined as patients with stage IV disease or stage III disease and > 1.0 cm residual disease after debulking surgery (18.1 months versus 14.5 months; p = 0.002); this translated to an increased median OS (36.6 months versus 28.8 months; p = 0.002). ● Perren T., Swart A., Pfisterer J., Ledermann J., Pujade-Lauraine E., Kristensen G., et al. for the ICON7 Investigators (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365: 2484–2496
- 13. Angiogenesis Inhibitor - CEDIRANIB ● Another angiogenesis inhibitor that is currently under investigation in ovarian cancer is cediranib. ● Cediranib is a tyrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR) -1, VEGFR-2, VEGFR-3, and c-kit.
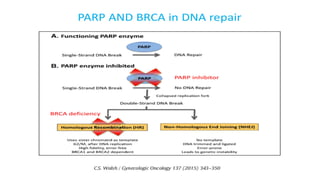
- 14. BRCA1/2 Mutation and PARP Inhibitors
- 21. Whom to test for BRCA mutation? Method of testing? ● All patients with a diagnosis of ovarian, fallopian tube, or peritoneal cancer should have a genetic risk evaluation, irrespective of their family history, as the results may impact treatment decisions. ● If germline testing is negative, we proceed with somatic BRCA testing to determine who may most benefit from a poly(ADP-ribose) polymerase (PARP) inhibitor. ● Relying only on somatic testing may miss up to 5 percent of germline mutations due to differences in test coverage, variant classification, and the inability to detect large rearrangements by available commercial next-generation sequencing(1). ● Relying only on germline testing will miss approximately 7 percent of patients who have somatic mutations only(2). 1. Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2018; 379:2495. 2. Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 2014; 20:764.
- 30. SUMMARY OF STUDY 19
- 31. THERAPIES CURRENTLY UNDER INVESTIGATION ● Vigil- autologous tumor vaccine (1) ● Chimeric antigen receptor T (CAR-T) cell immunotherapy(2) ● CA-125 Antibody- oregovomab (3) abagovomab(4) ● Dendritic Cells: Sotio DCVAC (5) ● Peptide: DIRAS3 and BECN1(6) ● Viral- adenovirus Onyx-015(7) ● Checkpoint Inhibitors- Cytotoxic T-lymphocyte Associated Protein 4 (CTLA-4), Programmed cell Death-1 (PD-1) and Programmed Death Ligand-1 (PD-L1). ● (1)Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B. et al. Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecologic Oncology. 2016;143:504–10. doi: 10.1016/j.ygyno.2016.09.018. ● (2) Yan W, Hu H, Tang B. Advances Of Chimeric Antigen Receptor T Cell Therapy In Ovarian Cancer. Onco Targets Ther. 2019;12:8015–22. doi: 10.2147/OTT.S203550. ● (3)Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. J Clin Oncol. 2009;27:418–25. doi: 10.1200/JCO.2008.17.8400. ● (4) Reinartz S, Kohler S, Schlebusch H, Krista K, Giffels P, Renke K. et al. Vaccination of patients with advanced ovarian carcinoma with the anti-idiotype ACA125: immunological response and survival (phase Ib/II) Clin Cancer Res. 2004;10:1580–7. doi: 10.1158/1078-0432.ccr-03-0056. ● (5) Cibula D, Mallmann P, Knapp P, Melichar B, Klat J, Minar L. et al. Dendritic Cell Vaccine Combined with Second Line of Chemotherapy in Patients With Epithelial Ovarian Carcinoma Final Analysis of a Phase II, Open Label, Randomized, Multicentre Trial. Journal of Clinical Oncology. 2018;36:e17515–6. ● (6) Sutton MN, Huang GY, Liang X, Sharma R, Reger AS, Mao W. et al. DIRAS3-Derived Peptide Inhibits Autophagy in Ovarian Cancer Cells by Binding to Beclin1. Cancers (Basel) 2019;11:557–71. ● (7) Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S. et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–9.
- 33. PAOLA-1 ● Stage III-IV disease with or without BRCA mutation ● 806 patients randomly assigned in a 2:1 ratio ● Olaparib tablets (300 mg twice daily) or placebo for up to 24 months; all patients received bevacizumab at 15 mg/kg every 3 weeks up to 15 months ● Median follow-up of 22.9 months ● Median PFS was 22.1 months vs 16.6 months [HR- 0.59]
- 35. NCCN GUIDELINES
- 36. DOSAGE OF BEVACIZUMAB IN MAINTENACE THERAPY ● ICON 7 STUDY 7.5MG/KG 15 MG/KG
- 37. BEVACIZUMAB ADVERSE REACTIONS 1. Gastrointestinal perforation and fistulae 2. Wound healing complications 3. Hemorrhage 4. Thromboembolic events 5. Hypertension 6. Renal toxicity and proteinuria 7. Infusion-related reaction 8. Posterior reversible encephalopathy syndrome (PRES
- 38. Olaparib Niraparib Veliparib Rucaparib Disease setting Stage III-IV BRCA-mutated Stage III-IV disease with or without BRCA mutation Stage III-IV disease with or without BRCA mutation Stage III-IV disease with or without BRCA mutation Dose 300mg BD 300mg OD 600mg BD Duration 2 yr 3 yr 2 yr 2 yr Monotherapy Yes Yes Yes Along with Bevacizumab (15mg/kg q3wkly 15 months) Yes Yes Trial SOLO-1[Phase III] PRIMA/ENGOT-OV26 VELIA/GOG-3005 trial ATHENA Control PFS :14 months 5 years- 21% ds free PFS [OP]- 8.2 month PFS[HRD] - 10.4 months PFS [OP]-17.3 month PFS[HRD] – 20.5 months PFS-[BRCA]- 22 month PFS [OP]- 9.2 month PFS[HRD] - 11.3 months PFS-[HRP]-9.1 month Test arm PFS : 56 months 5 yr – 48% ds free PFS[0P] – 13.8 month PFS[HRD]- 21.9 month Gain of 2.7 months in HRD neg PFS [OP]-23.5 month PFS[HRD] – 31.9 months PFS-[BRCA]-35 month PFS [OP]-20.2 month PFS[HRD] – 28.7 months PFS-[HRP]-12.1 month HR (95% Cl) PFS HR: 0.30 PFS HR: 0.62 HRD PFS HR: 0.43 PFS [BRCA] HR 0.44 PFS [op] HR 0.52 PFS [HRD] HR 0.47 PFS [HRP] HR 0.65
- 39. SAFETY PROFILES OF THE DIFFERENT PARP INHIBITORS
- 40. ESMO Guidelines ● Overall survival benefit in BRCAmut ovarian cancer has led to a change in clinical practice ● Testing for germline and somatic BRCAmut is now part of routine clinical practice ● Tumours with Homologous Recombination Repair deficiency beyond BRCA also benefit from PARPi therapy ● Recurrence still occurs in ~50% of women with BRCAmut either on or after PARPi therapy ● New and better treatments are still needed to further improve outcomes Research needed to explore how best to treat women with relapsed ovarian cancer after first-line maintenance treatment with a PARPi
- 41. ESMO Guidelines ● In newly diagnosed ovarian cancer, a PARPi, with or without bevacizumab, is recommended for patients with BRCA1/2- mutated or BRCA1/2-wt/HRD- positive tumours with no evidence of disease at the end of chemotherapy, or a complete or partial response to platinum–paclitaxel first-line ● For BRCA1/2-mutated: olaparib for 2 years; niraparib for 3 years or olaparib–bevacizumab for 2 years; rucaparib 2 years ● For BRCA1/2-wt- HRD-positive: niraparib for 3 years; olaparib– bevacizumab for 2 years; rucaparib 2 years ● HRD-negative tumours: bevacizumab or niraparib for 3 years, or rucaparib for 2 years
- 42. THANK YOU






























![PAOLA-1
● Stage III-IV disease with or without BRCA mutation
● 806 patients randomly assigned in a 2:1 ratio
● Olaparib tablets (300 mg twice daily) or placebo for up to 24 months; all
patients received bevacizumab at 15 mg/kg every 3 weeks up to 15 months
● Median follow-up of 22.9 months
● Median PFS was 22.1 months vs 16.6 months [HR- 0.59]](https://image.slidesharecdn.com/carcinomaovary-maintenancetherapy-250221085418-02e227ae/85/Carcinoma-Ovary-maintenance-therapy-pptx-33-320.jpg)




![Olaparib Niraparib Veliparib Rucaparib
Disease setting Stage III-IV
BRCA-mutated
Stage III-IV disease
with or without
BRCA mutation
Stage III-IV disease
with or without
BRCA mutation
Stage III-IV disease
with or without
BRCA mutation
Dose 300mg BD 300mg OD 600mg BD
Duration 2 yr 3 yr 2 yr 2 yr
Monotherapy Yes Yes Yes
Along with
Bevacizumab (15mg/kg
q3wkly 15 months)
Yes Yes
Trial SOLO-1[Phase
III]
PRIMA/ENGOT-OV26 VELIA/GOG-3005 trial ATHENA
Control PFS :14 months
5 years- 21% ds
free
PFS [OP]- 8.2 month
PFS[HRD] - 10.4 months
PFS [OP]-17.3 month
PFS[HRD] – 20.5 months
PFS-[BRCA]- 22 month
PFS [OP]- 9.2 month
PFS[HRD] - 11.3 months
PFS-[HRP]-9.1 month
Test arm PFS : 56 months
5 yr – 48% ds
free
PFS[0P] – 13.8 month
PFS[HRD]- 21.9 month
Gain of 2.7 months in
HRD neg
PFS [OP]-23.5 month
PFS[HRD] – 31.9 months
PFS-[BRCA]-35 month
PFS [OP]-20.2 month
PFS[HRD] – 28.7 months
PFS-[HRP]-12.1 month
HR (95% Cl) PFS HR: 0.30 PFS HR: 0.62
HRD PFS HR: 0.43
PFS [BRCA] HR 0.44 PFS [op] HR 0.52
PFS [HRD] HR 0.47
PFS [HRP] HR 0.65](https://image.slidesharecdn.com/carcinomaovary-maintenancetherapy-250221085418-02e227ae/85/Carcinoma-Ovary-maintenance-therapy-pptx-38-320.jpg)



