Developing Protocols & Procedures for CT Data Integrity
- 1. PROTOCOLS & PROCEDURES FOR CLINICAL TRIAL DATA INTEGRITY Dr. Bhaswat S. Chakraborty Senior Vice President, R&D Cadila Pharmaceuticals Ltd. 1 Presented at the International Conference on CRO/Sponsor Summit on “Data Integrity in Clinical Research” at Hyderabad, India, 21-22 July, 2016
- 2. CONTENTS Clinical trial data Approaches to build and maintain data integrity CT Data management system On-site monitoring Centralized monitoring Alternate monitoring Risk based monitoring (RBM) Data & safety monitoring plan (DSMP) Data & safety monitoring board (DSMB) Training & inspection-readiness Concluding remarks 2
- 3. Investigational Sites Product Management Project Management Drug & Clinical Trial Development Extended Picture IRB Regulatory Documents Relationship Building eMails Partners & Affiliates Meetings CROs Contracts Knowledge Information Safety Communication Resource Management Data Capture Data Management Multidirectional Flow of Data and Decisions 3
- 4. CLINICAL TRIAL DATA AND DOCUMENTS Study and site feasibility documents Protocol Inclusion/Exclusion criteria Informed Consent Investigators brochure Training documents and data Data Randomisation, Blinding CRF/ECRF (Demographics and site visit data) Primary and secondary outcome variables (end points) Clinical procedures and study conduct data Investigational products: Supply, Inventory, Handling & Usage, Retention Safety monitoring and signal detection Subject withdrawal and retention data Data and safety monitoring committee (activities, data, reports) Data management and data monitoring including SDV by Sponsor/CRO Data recording and reporting Statistical analysis Study reporting .. 4
- 5. WHY DO WE NEED A DATA MANAGEMENT & DATA INTEGRITY SYSTEM? Enormous volumes of data Example, a Phase-III trial in 10 centres with 100 patients each 60 pages of CRF for each recruited patient 20 fields each page 40 pages of screening form for each candidate patient 20 fields each page [1000 (60 x 20)] + [1500 (40 x 20)] = 12, 00000 + 12, 00000 = 24,00000 specific data points 5
- 6. CLINICAL TRIAL DATA Useful only if it is clean & accurate Data processing must be real-time subject randomization management of clinical trials materials laboratory uploads patient diary data Integrated Consistent Accurate Data structures must be Standard Validated Data transfer method must be Standard Validated 6
- 7. DATA INTEGRITY IN RESEARCH Research integrity depends on data integrity Includes all aspects of collection, use, storage and sharing of data. Data integrity is a shared responsibility Everyone involved in the research is responsible. The ultimate responsibility belongs to the PI. However, there is a broader role and responsibility for the institute and scientific community. Transparency of the research data is required 7 Free and accurate information exchange is fundamental to scientific progress Van Eyk J., JHU NHLBI Innovative Proteomics Center on Heart Failure
- 8. SOURCES OF DATA INTEGRITY & ITS LACK Data integrity is based on accurate and traceable: Collection Organization & Storage Analysis Reporting Data integrity can be compromised numerous ways: Malicious or unethical intentions Human mistakes and naivety Technical error Misinterpretation 8 Accurate & complete data = High quality data = QbD & RBM data = IRB+DSMP+DSMB data
- 9. CONSEQUENCES OF NON- INTEGRITY Personal loss Blocked scientific progression Impaired technology development Damage to the institution, sponsors or CRO Tarnished public perception of science Damage to or loss of patent protection 9 Van Eyk J., JHU NHLBI Innovative Proteomics Center on Heart Failure
- 10. APPROACHES TO BUILD AND MAINTAIN DATA INTEGRITY Monitoring, monitoring ,,,, monitoring CTDM Systems On site monitoring Centralized monitoring Clinical trial quality assurance units (QAUs) Sponsors often use internal or external QAUs Not required by regulation QbD and Risk based monitoring Building QbD Risk identification & assessment Critical attributes and riskcategorization thereof Plans and processes Targeted monitoring 10
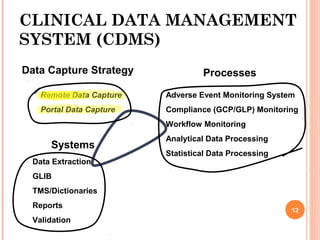
- 11. CLINICAL DATA MANAGEMENT SYSTEM (CDMS) Data Capture Strategy Remote Data Capture Portal Data Capture Processes Adverse Event Monitoring System Compliance (GCP/GLP) Monitoring Workflow Monitoring Analytical Data Processing Statistical Data Processing Systems Data Extraction GLIB TMS/Dictionaries Reports Validation 12
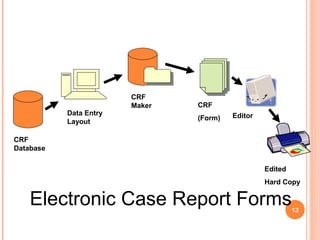
- 12. CRF Database Data Entry Layout CRF Maker CRF (Form) Editor Edited Hard Copy Electronic Case Report Forms13
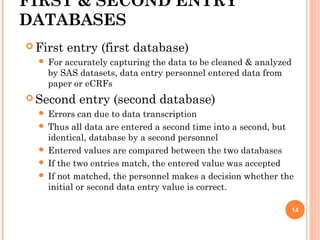
- 13. FIRST & SECOND ENTRY DATABASES First entry (first database) For accurately capturing the data to be cleaned & analyzed by SAS datasets, data entry personnel entered data from paper or eCRFs Second entry (second database) Errors can due to data transcription Thus all data are entered a second time into a second, but identical, database by a second personnel Entered values are compared between the two databases If the two entries match, the entered value was accepted If not matched, the personnel makes a decision whether the initial or second data entry value is correct. 14
- 14. CLINICAL DATA MANAGEMENT SYSTEM (CDMS).. Data Capture Strategy Remote Data Capture Portal Data Capture Processes Adverse Event Monitoring System Compliance (GCP/GLP) Monitoring Workflow Monitoring Analytical Data Processing Statistical Data Processing Systems Data Extraction GLIB TMS/Dictionaries Reports Validation
- 15. Raw Data DATA EXTRACTION, CLEANING & LOCKING Real time query Are the queries answered? Yes No Data cleaning 1. Detecting & diagnosing errors 2. Editing incorrect data 3. Integrated data passage 4. Outlier determination 5. Robust estimation of analytical parameters Clean data Approval required Can this data be locked? Yes NoRepeat Observation/ Omission Locked data Analysis
- 16. ON-SITE MONITORING FOR INTEGRITY Effective monitoring is critical to: Human subject protection Conduct of high quality studies‐ Data Integrity On-site monitoring An in-person evaluation at the investigational sites Can identify data entry errors missing data in source records or CRFs provide assurance that study documentation exists assess compliance with the protocol and investigational product quality of the overall conduct of the trialat that site particularly helpful early in a study, especially if protocol is complex and includes novel procedures lead to meaningful training efforts 17
- 17. CENTRALIZED MONITORING A remote evaluation carried out by sponsor personnel or CRO By clinical monitors, data management personnel, or statisticians At a location other than the sites Can provide many of the capabilities of on-site monitoring as well as value additions Success of centralized monitoring depend on various factors Use of electronic systems Access to subjects’ electronic records Timeliness of data entry from paper CRFs Must ensure that record keeping, data entry, and reporting and supporting source data are well-defined & accessible Must also identify in their monitoring plan when one or more on-site monitoring visits are required 18 Alternative monitoring techniques can exist
- 18. ALTERNATE MONITORING Monitor/review data quality missing data, inconsistent data, data outliers, and potential protocol deviations Conduct statistical analyses to identify data trends not easily detected by onsite monitoring, such as checks of range, consistency, completeness, unusual distribution of data within and between sites Analyze site characteristics, performance metrics high screen failure, withdrawal rates, high eligibility violations, delays in reporting .. Verify critical source data remotely where accessible; CRF data are according to the protocol? Complete administrative and regulatory tasks IRB approvals, IP accountability, randomization and CRF data, previously requested CRF corrections are made Communication with Study Site Staff Teleconferences, videoconferencing, email Review site’s processes, procedures, and records technique 19
- 19. RISK-BASED MONITORING Basis: Monitoring activities prevent or mitigate important and likely sources of error in conduct, collection, and reporting of critical data and processes necessary for human subject protection and trial integrity Importance of Critical Quality Factors: Procedures critical to collecting reliable data for study endpoints Consistency across sites or in a highly specific manner in some sites Procedures that won’t significantly impact data analysis or subject safety 1. Identify Critical Data and Processes to be Monitored: IC verification, adherence to protocol eligibility criteria, accountability and administration of IP, conduct, documentation & assessments related to study endpoints & red safety assessments Procedures essential to trial integrity, e.g., blinding is maintained, both at the site level and at the sponsor level 20 Some types of errors in CT is more important than others (error in age v/s error in endpoint)
- 20. RISK-BASED MONITORING.. 2. Risk Assessment: Risk identification based on trial design or investigational product Risks assessed and prioritized by considering the following: the likelihood of errors occurring the impact of such errors on human subject protection and trial integrity the extent to which such errors would be detectable 3. Factors to consider while developing a monitoring plan: Complexity of the study design may require increased frequency and extent of review (adaptive designs, stratified designs, complex dose titrations..) 4. Monitoring Plan: Description of each monitoring method & how it will be used to address important risks and ensure the validity of critical data Criteria for determining the timing, frequency, and extent of planned monitoring activities 5. Documentation of monitoring: Date of the activity and the individual(s) conducting and participating in it Summary of the data or activities reviewed Description of noncompliances, potential noncompliance, data irregularities.. A description of any actions taken 21 Use the results of risk assessment in developing monitoring plan and type and intensity of monitoring to address this risks
- 21. DATA & SAFETY MONITORING PLAN DECISION TREE 22 Clinical Research? Yes No Clinical Trial? Phase III or Multicenter Blinded, high risk interventions or vulnerable populations Yes No IRB Yes No IRB + DSMP IRB + DSMP + DSMB Yes No Source: NIDA
- 22. MONITORING REQUIRED IN DIFFERENT PHASES OF STUDY OF CLINICAL TRIAL Phase I trial involves relatively high risk to a small N Usually the study investigator performs continuous monitoring of safety Phase II trial follows phase I with more N Toxicity and outcomes are confounded by disease process Monitoring similar to that of a phase I trial or additional monitoring by experts or DSMB Phase III trials frequently compares a new treatment to a standard treatment or to no treatment Large N Short-term risk is low, but long term effects of IP to achieve significant safety or efficacy difference from the control May require a DSMB to perform monitoring functions 23
- 23. DATA AND SAFETY MONITORING PLAN (DSMP) & BOARD (DSMB) DSMPs promote data integrity as well as safety and protection of trial subjects Many common activities with other trials and some unique activities for a particular trial depending on the risk, size and complexity Subject safety, data integrity and validity are the foremost consideration for a DSMP For low risk studies an annual safety monitoring (by PI) may be enough For higher risks studies, safety monitoring will have to be done more frequently as required by an established DSMB/DMC Usually DSMBs are not required for Phase I studies but only for Phase II and III, but exceptions exists 24
- 24. WHEN DO YOU NEED A DSMB? When do you need a DSMB? To ensure that participants are not exposed to undue risk To ensure that the study will yield usable results To do Interim Analyses and/or change protocol study design based on IA To maintain trial integrity To ensure unbiased, timely publication of results Composition: Researcher(s)/Clinician knowledgeable of the science of the study Biostatistician and/or Epidemiologist (study design/analysis) Research subject advocate and/or ethicist (research ethics) SOP The DSMB chairman and members (quorum) participate Frequency at which the study will be evaluated, e.g. annually, quarterly etc. Adverse event reporting requirements A description of interim efficacy analyses, if appropriate Study stopping rules, if appropriate The distribution of DSMB reports 25
- 25. DATA AND SAFETY MONITORING PLAN (DSMP) The specifics of a DSMP depend upon the nature, size, complexity, and risk level To reduce harm or injury to study individuals The minimum required content for a DSMP includes: Assessment of levels of risk A plan for safety review (by whom and how often) Anticipated adverse events AE grading and attributions A plan for unanticipated and/or SAE reporting A plan for periodic or annual reporting of AEs Ensure compliance with the principles and process of IC Assessment of protocol compliance including violations and/or deviations A plan for compliance with privacy related regulations (e.g., HIPAA) 26
- 26. TRAINING OF CLINICAL INVESTIGATORS & DATA PROCESSORS/ANALYSTS Clinical trial monitors train the CI and site staff during a study Training on the conduct of a study and appropriate instruction during the study (e.g., when changes are made to the protocol) Teleconferences, webcasts, online training modules and communication methods for training and feedback & significant change notification Sponsors who plan less frequent monitoring should consider Monitoring plus discussion of CI’s and site staff’s responsibilities, feedback, and additional training Train the trainers, data managers, analysts & report writers Delegating monitoring to a CRO Need written transfer of any obligations from a sponsor to a CRO & CRO requires to comply with the regulations Sponsors should evaluate CRO compliance with regulatory requirements & contractual obligations e.g., periodic review of monitoring reports and vendor performance or quality metrics, communication between the sponsor & CRO regarding monitoring progress and findings Sponsors are ultimately responsible for all data & monitoring 27
- 27. PREPARING AS A SPONSOR OR A CRO FOR FDA AUDIT Registration of studies on http://www.clinicaltrials.gov/ For CTs of drugs and biologics other than Phase-I Selection and monitoring of clinical investigators TMF & SMFs Sponsor/CRO provide the investigators with all necessary information prior to initiation of CT Serious deviations from the approved investigational plan (which includes the study protocol) or FDA regulations Terminated sites and the circumstances Non-compliant investigator still continuing in the study Selection of Monitors Criteria for selecting monitors and how monitors meet those criteria How more than one person handles monitoring Monitoring procedures and activities Procedures, frequency, scope, and process to monitor the progress of the clinical investigation Relevant SOPs are followed Activities Review of Site Records 28
- 28. PREPARING AS A SPONSOR OR A CRO FOR FDA AUDIT.. QAUs (CT QAUs are not required by regulation) However, if the sponsor/CRO has a QAU, its organization & operation should be described in SOPs; separation of functions between QA auditing and monitoring of clinical trials List of audited studies Safety/AE reporting Safety reports of potential serious risks within 15 calendar days; within 7 calendar days if unexpected fatal or life-threatening suspected AE AE reported to participating investigators (and to reviewing IRBs for device studies) as required by the regulations. Procedures (e.g., frequency, scope) the sponsor/CRO uses for the receipt, evaluation, and monitoring of safety information/unanticipated AEs All Study Tabulations All Investigator Tabulations All pertinent studies (form 1572) included in the above two categories Reasons for non-inclusion Data tabulations on each subject in each clinical trial in an NDA 29
- 29. PREPARING AS A SPONSOR OR A CRO FOR FDA AUDIT ... Electronic records and electronic signatures Regulatory requirements for the clinical data are the same regardless of the type of computer system used by the clinical site Data must be reliable and usable for evaluating the safety and/or effectiveness of FDA-regulated products Electronic Records (CFR Part 11) and electronic signatures are maintained in electronic format (in some cases in addition to paper format); SOPs for procedures Data collection: Procedures for collection, retention, and transmission of data at each clinical site; File transfer protocols to and from processing center to and from site Original data entries and changes cannot be made by anyone other than the clinical investigators How electronic data are reviewed during monitoring visits; SOPs for such reviews Data Security; Clear, justified authority to access the system How the paper & computerized systems are accessed (e.g., password protected, access privileges, user identification How information is captured related to the creation, modification, or deletion of electronic records (e.g., audit trails, date/time stamps); backup, disaster recovery, and/or contingency plans; error messages or system failures handled/corrected How the system and data are handled during site closure 30
- 30. CONCLUDING REMARKS A CT is as good as the quality of its data In an effort to ensure the integrity of clinical trial data, the FDA has released requirements Monitoring of data collection, review and analysis is essential to ensure data integrity Traditionally monitoring required an in-depth and comprehensive examination of all collected data. The risk-based monitoring (RBM) system is fundamentally different as to how data managers review clinical data Does not mandate a specific methodology but requireds an ideal strategy that allows for faster time to market, reduces site monitoring costs and frees up time and resources for value-added tasks For complex Phase III (sometimes Phase II) trials require a DSMP as well as a DSMB for overall data integrity or to stop the trial Training and audit (FDA/Client) readiness for data integrity ensures high success rates 31
Editor's Notes
- #16: GLIB: global library is an organization wide central repository for containing standardized data definitions. TMS: thesaurus management system; e.g., Oracle TMS provides terminology services for Oracle Clinical, Oracle Remote Data Capture, Oracle Adverse Event Reporting System, and Oracle Life Sciences Data Hub. Allows access to any number of dictionaries, including multiple versions of the same dictionary ; supports any number of hierarchy levels and supports custom or commonly used dictionaries, such as MedDRA, MedDRAJ, MedDRA SMQs, SNOMED, ICD9, WHO-ART, and WHO-Drug. MedDRA or Medical Dictionary for Regulatory Activities is a clinically validated international medical terminology used by regulatory authorities and the regulated biopharmaceutical industry during the regulatory process, from pre-marketing to post-marketing activities, and for data entry, retrieval, evaluation, and presentation. In addition, it is the adverse event classification dictionary endorsed by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA is used in the United States, European Union, and Japan. Its use is currently mandated in Europe and Japan for safety reporting.
- #20: Bullet 1 - that may be indicative of systemic or significant errors in data collection and reporting at a site





![WHY DO WE NEED A DATA
MANAGEMENT & DATA INTEGRITY
SYSTEM?
Enormous volumes of data
Example, a Phase-III trial in 10 centres with
100 patients each
60 pages of CRF for each recruited patient
20 fields each page
40 pages of screening form for each candidate
patient
20 fields each page
[1000 (60 x 20)] + [1500 (40 x 20)]
= 12, 00000 + 12, 00000
= 24,00000 specific data points 5](https://image.slidesharecdn.com/developingprotocolsproceduresforctdataintegrityfinalfinal-170302043711/85/Developing-Protocols-Procedures-for-CT-Data-Integrity-5-320.jpg)