DUR and DUE.ppt
- 1. Drug Utilization Review & Drug Utilization Evaluation: An Overview
- 2. Definition of DUR and DUE • Drug Utilization Review (DUR): An authorized, structured, ongoing review of health care provider prescribing, pharmacist dispensing, and patient use of medication. • Drug Utilization Evaluation (DUE): A qualitative evaluation of drug use, prescribing, and member fill patterns to determine the appropriateness of drug therapy. It also provides a mean of correcting problems thus contributing to RUD.
- 3. Objectives of DUR and DUE • Ensuring drug therapy meets the current standards of care • Improve quality of care and overall drug effectiveness • Prevent adverse drug reactions • Encourage the practice of evidence-based, clinically appropriate, cost-effective drug use • Reduce drug misuse and abuse • Reduce costs related to inappropriate drug use • Identifying areas requiring further education of health care practitioners
- 4. Medication use evaluation MUE • Similar to DUE, emphasizes on improving patient outcomes and individual’s Qol • MUE will assess clinical outcomes e.g. cured infections, decreased/ increased B.P, decreased lipid levels etc. Additional objectives may include • Creating guidelines for appropriate drug utilization • Evaluating effectiveness of medical therapy • Controlling medicine cost • Areas for further education of HCPs
- 5. Pharmacist Role in DUR & DUE • Identifies opportunities for quality improvement • Participates in efforts to improve: – Patient outcomes – Quality of programs • Promotes appropriate drug use to reduce overall health care costs and improve access to care • Carries out ethical and professional responsibility
- 6. A Model DUR Program • Access to member drug utilization data • Qualified pharmacists with authority to review • Knowledge of population served and delivery system • Availability of established standards for comparison • Measurement of utilization review outcomes
- 7. Prospective DUR • A screening method by which a health care provider reviews the necessity of drug therapy before it is dispensed or administered – Electronic DUE programs at retail pharmacies – Prior authorization (PA) programs – Drug-drug and drug-disease interactions – Dosing appropriateness – Drug-patient precautions (due to age, allergies, gender, pregnancy, etc.) – Medication directions – Formulary substitutions (e.g., therapeutic interchange, generic substitution) – Inappropriate duration of drug treatment
- 8. Prospective DUR Issues Commonly Addressed by Prospective DUR 1- Clinical abuse/misuse 2- Drug-disease contraindications (when a prescribed drug should not be used with certain diseases) 3- Drug dosage modification 4- Drug-drug interactions (when two or more different drugs interact and alter their intended effects, often causing adverse events) 5- Drug-patient precautions (due to age, allergies, gender, pregnancy, etc.) 6- Formulary substitutions (e.g., therapeutic interchange, generic substitution) 7- Inappropriate duration of drug treatment
- 9. Concurrent DUR • A screening method by which a health care provider reviews the necessity of drug therapy at the time of dispensing or during treatment – Case management – Review of patient records – Research projects that follow patients in randomized, controlled trials – Real-time system edits at the point of service – Over or underutilization of medication – Excessive or insufficient dosing – Drug-drug interactions – Drug-disease interactions – Drug dosage modifications
- 10. Concurrent DUR Issues Commonly Addressed by Concurrent DUR 1- Drug-disease interactions 2- Drug-drug interactions 3- Drug dosage modifications 4- Drug-patient precautions (age, gender, pregnancy, etc.) 5- Over and underutilization 6- Therapeutic Interchange
- 11. Retrospective DUR • A screening method by which a health care provider reviews the necessity of drug therapy after it has been dispensed or treatment has started – Review of medical charts, electronic medical records and/or claims data to assess appropriate drug use – Review provider prescribing patterns – Quality assurance analyses – Developing standard guidelines to achieve target outcomes at a population level – Appropriate generic use – Use of formulary medications whenever appropriate – Therapeutic appropriateness and/or duplication
- 12. Retrospective DUR Issues Commonly Addressed by Retrospective DUR 1- Appropriate generic use 2- Clinical abuse/misuse 3- Drug-disease contraindications 4- Drug-drug interactions 5- Inappropriate duration of treatment 6- Incorrect drug dosage 7- Use of formulary medications whenever appropriate 8- Over and underutilization 9- Therapeutic appropriateness and/or duplication
- 13. The DUR Process 1. Determine criteria – The criteria should focus on relevant outcomes within a delineated scope for DUR and identify the relevant drugs to be monitored for optimal use 2. Collect data – Measure the actual use of medications (purchasing record, issue records, prescription records 3. Compare the data to established criteria – Involves applying the algorithm, identifying members who meet the DUR criteria and the comparison between optimal or appropriate and actual use 4. Perform intervention – Action should be targeted to areas of concern such as prescribing patterns, medication misadventures, and quality of drug therapy or economic consideration.
- 14. The DUR Process 5. Analyze results – Evaluate the outcomes and document reasons for positive and negative results 6. Document DUR – Report the findings to the appropriate team within the organization (e.g., the pharmacy & therapeutics committee) and/or individual prescribers when appropriate 7. Re-evaluate the program (on-going)
- 15. The DUR Process WHO Guidelines/steps of DUE STEP 1: Establish responsibility • It is the responsibility of the DTC to establish procedures for the implementation of a DUE program; this includes appointing a responsible member of the DTC or a subcommittee to monitor and supervise the DUE process in the hospital or clinics. Ideally the DTC should establish annual plans, outlining which medicines or clinical conditions will be a part of the DUE process.
- 16. The DUR Process WHO Guidelines/steps of DUE STEP 2: Develop the scope of activities and define the objectives The DTC should decide upon the objectives of the DUE and the scope of the activities necessary. The scope can be very extensive or it can focus on a single aspect of drug therapy and will depend upon the type of problem identified, for example: • overuse of a more expensive medicine when a cheaper equivalent is available, as revealed in aggregate data • incorrect use (indication, dosage, administration) of a particular drug, as revealed in patient charts, medication error reports, ADR reports • inappropriate choices of antibiotic, as revealed in antibiotic sensitivity reports • a poor dispensing process, as revealed by patient complaints or feedback.
- 17. The DUR Process WHO Guidelines/steps of DUE Selection of Drugs for DUE • Due to the large number of medicines available at a hospital or clinic, the DTC must concentrate on those medicines with the highest potential for problems in order to get the most return on the work involved. These high-priority areas include: • high-volume drugs • expensive drugs • drugs with a narrow therapeutic index • drugs with a high incidence of ADRs • critically important therapeutic categories, for example cardiovascular, emergency, toxicology, intravenous drugs, chemotherapy and narcotic analgesics • antimicrobial drugs, prophylactic and therapeutic • drugs undergoing evaluation for addition to the formulary • drugs used for non-labelled indications • drugs used in high-risk patients • common clinical conditions often poorly treated.
- 18. The DUR Process WHO Guidelines/steps of DUE STEP 3:Establish criteria for review of the medicine Establishing DUE criteria is extremely important, and is the responsibility of the DTC. • DUE criteria are statements that define correct drug usage with regard to various components. Criteria for the use of any medicine should be established using the hospital’s STGs (assuming that they have been correctly developed). • In the absence of hospital STGs, criteria may be based on recommendations from national or other locally available satisfactory drug use protocols, other relevant literature sources, and/or recognized international and local experts. • Credibility, and staff acceptance, of the DUE relies on using criteria that have been developed from reading established evidence-based medicine information from reputable sources and that have been discussed with prescribers.
- 19. The DUR Process WHO Guidelines/steps of DUE COMPONENTS OF DRUG USE FOR DUE CRITERIA • uses: appropriate indication for drug, absence of contraindications • selection: appropriate drug for clinical condition • dosing: indication-specific dosing, intervals and duration of treatment • interactions: absence of interactions - drug-drug, drug-food, drug- laboratory • preparation: steps involved with preparing a drug for administration • administration: steps involved in administration, quantity dispensed • patient education: drug and disease-specific instructions given to patients • monitoring: clinical and laboratory • outcome, for example: decreased blood pressure, blood glucose, asthma attacks
- 20. The DUR Process WHO Guidelines/steps of DUE STEP 4:Data collection • Data may be collected retrospectively, from patient charts and other records, or prospectively, at the time a medicine is prepared or dispensed. • The treatment of at least 30 patients, or 100 patients for common clinical conditions, should be reviewed per health facility or hospital.
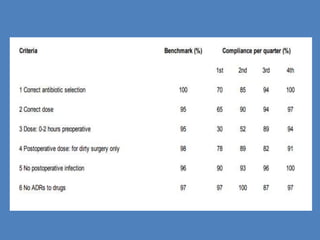
- 21. The DUR Process WHO Guidelines/steps of DUE STEP 5:Data Analysis • Data are tabulated in a form that corresponds to the criteria chosen for the DUE. The percentages of cases that meet the threshold for each criteria should be calculated and summarized for presentation to the DTC. A report of all DUE programmes that are being conducted should be prepared on a quarterly basis.
- 22. The DUR Process WHO Guidelines/steps of DUE STEP 6: Feedback to the prescribers and making a plan of action After information is presented (for example on inappropriate drug use or unacceptable patient outcome), the DTC should develop conclusions about the differences between actual and desired results. • In other words, how do the actual results vary from the desired benchmark or threshold levels? • The DTC should then decide what follow-up action is necessary and whether to continue, discontinue or expand the functions of the DUE in question. Recommendations should include specific steps to correct any drug use problem that is evident from performing the DUE. • For example, if a specific medicine is being prescribed at too high a dose, the recommendations need to specify in detail how the dosing of this medicine can be improved. Interventions to improve drug use would include feedback to the prescribers
- 23. The DUR Process WHO Guidelines/steps of DUE STEP 7: Follow-up In every DUE, follow-up is critical to ensure appropriate resolution of any problems. • Did an intervention achieve its objective? • If an intervention is not evaluated, or drug use problems are not resolved, then the DUE will have been of no use. • As a part of a follow-up plan the DTC must assess the need to continue, modify or discontinue the DUE. • Thus, DUE activities should be evaluated regularly (at least annually) and those that do not have a significant impact on drug use should be redesigned in order to provide measurable improvements. • Common problems associated with DUEs include unclear responsibilities for different activities, poor prioritization of problems, lack of documentation, lack of personnel and inadequate follow-up. • If follow-up is adequate, prescribers are likely to improve their performance in all areas knowing that they may be reviewed in the future!
- 24. Who Benefits from DUE/DUR? • Plan member • Health care provider • Pharmacist • Health care system
- 25. Example "This asthma is really slowing me down. This prescription isn't helping much." • Example Scenario: Tim's asthma is not well controlled, and he uses his inhaler multiple times a day. Tim's therapy should most likely be increased to prevent further medical complications. • Pharmacist Interaction: A pharmacist conducted concurrent DUR at the health plan and noticed that Tim was only prescribed an as-needed inhaler. With the pharmacist's recommendation to the prescriber, derived from evidence-based guidelines, Tim was prescribed a maintenance asthma medication. • Benefit: Although another medication was added, the patient and the health plan have an overall cost savings. The added prescription vastly decreases Tim's likelihood of a costly emergency room visit for a severe asthma attack and enhances Tim's quality of life.
- 26. Conclusion • A pharmacist performs DUR/DUE to improve overall access and quality of care, and to reduce costs • Each type of DUR represents an important step in ensuring that the member receives the most appropriate, cost-effective medication • A successful DUR/DUE program benefits all health care players, including the member
Editor's Notes
- #3: DUR: Also referred to as medication use management; Cost – is the member using the most appropriate, least costly medication? Is adherence affected if the patient cannot afford the medication? Safety – is the member using a medication or does the member have a condition that interacts with the newly requested medication? Does the member have a gene that predisposes him/her to a particular fatal reaction/response? Safety considerations also include abuse/misuse. Efficacy – is the member using the most effective drug combination? most effective, least costly medication? Does the member have a gene that ensures a positive response to the treatment? DUE: Also referred to as medication use evaluation (MUE). Evaluation of drug use over time which often requires a multidisciplinary effort.
- #4: DUR and DUE are quality assurance methods that are viewed as such by accrediting and quality assuring bodies such as the Joint Commission on Accreditation of Health Care Organizations (JCAHO) for hospitals and National Committee for Quality Assurance (NCQA) for health plans, for example.
- #7: The DUR model originated in 1976 and its proposed structure is still used today. Now, the greatest emphasis in DUR program design is that DUR programs are “ongoing” or “continuous” which requires perpetual evaluation, communication, and adaptation through corrective actions to ensure a quality program.
- #8: This process allows the pharmacist to identify and resolve problems before the patient has received the medication. Pharmacists routinely perform prospective reviews in their daily practice by assessing a prescription medications dosage and directions while reviewing patient information for possible drug interactions or duplicate therapy. Upon reviewing the patient's prescriptions, the pharmacist would note the potential drug interaction and contact the prescriber to alert him/her to the problem. Example: Identification of drug-drug interactions are a common outcome of a prospective DUR. For example, a patient being treated with warfarin to prevent blood clots may be prescribed a new drug by another specialist to treat arthritis. If taken together, the patient could experience internal bleeding.
- #9: This process allows the pharmacist to identify and resolve problems before the patient has received the medication. Pharmacists routinely perform prospective reviews in their daily practice by assessing a prescription medications dosage and directions while reviewing patient information for possible drug interactions or duplicate therapy. Upon reviewing the patient's prescriptions, the pharmacist would note the potential drug interaction and contact the prescriber to alert him/her to the problem. Example: Identification of drug-drug interactions are a common outcome of a prospective DUR. For example, a patient being treated with warfarin to prevent blood clots may be prescribed a new drug by another specialist to treat arthritis. If taken together, the patient could experience internal bleeding.
- #10: Concurrent DUR is typically conducted jointly by a direct care/care coordination provider and a non-care provider (ex: nurse case manager and dispensing pharmacist). Some authors view “point-of-sale” edits as concurrent DUR. Examples include: drug interaction, drug allergy, inappropriate dose, and duplicate therapy alerts. Example: Concurrent DUR often occurs in institutional settings, where patients often receive multiple medications. Periodic review of patient records can detect actual or potential drug-drug interactions or duplicate therapy. It can also alert the pharmacist to the need for changes in medications, such as antibiotics, or the need for dosage adjustments based on laboratory test results. The key prescriber(s) must then be alerted to the situation so corrective action can be taken.
- #11: Concurrent DUR is typically conducted jointly by a direct care/care coordination provider and a non-care provider (ex: nurse case manager and dispensing pharmacist). Some authors view “point-of-sale” edits as concurrent DUR. Examples include: drug interaction, drug allergy, inappropriate dose, and duplicate therapy alerts. Example: Concurrent DUR often occurs in institutional settings, where patients often receive multiple medications. Periodic review of patient records can detect actual or potential drug-drug interactions or duplicate therapy. It can also alert the pharmacist to the need for changes in medications, such as antibiotics, or the need for dosage adjustments based on laboratory test results. The key prescriber(s) must then be alerted to the situation so corrective action can be taken.
- #12: Ties to drug utilization evaluation for identifying trends and adapting approval criteria based on evaluation of the impact of such criteria on drug use. Example: An example of a retrospective DUR may be the identification of a group of patients whose therapy does not meet approved guidelines. For example, a pharmacist may identify a group of patients with asthma, who according to their medical and pharmacy history, should be using orally inhaled steroids. Using this information, the pharmacist can then encourage prescribers to utilize the indicated drugs.
- #13: Ties to drug utilization evaluation for identifying trends and adapting approval criteria based on evaluation of the impact of such criteria on drug use. Example: An example of a retrospective DUR may be the identification of a group of patients whose therapy does not meet approved guidelines. For example, a pharmacist may identify a group of patients with asthma, who according to their medical and pharmacy history, should be using orally inhaled steroids. Using this information, the pharmacist can then encourage prescribers to utilize the indicated drugs.
- #25: Member – receives most appropriate therapy HC Provider – improved quality care for their patients; knowledge of how they compare to fellow providers and someone there to assure quality; unbiased education on standards of care/practice delivery Pharmacist – supports DUR activities on the bench esp for pharmacists who struggle to do a better job at this Health care system – better control of drug costs– greatest contributor to expenditure on health care in the nation.