final_acid_base_balane
- 1. ACID BASE BALANCE OR Homeostasis of Blood pH OR Regulation of Blood pH Dr Anissa Atif Mirza
- 2. Synopsis • Introduction • Sources of Acids and Bases in body • What is Acid Base Balance? • Mechanisms Regulating Blood pH. • Significance of Maintaining Acid Base Balance • Acid Base Imbalance and their conditions. • Diagnostic Tests
- 3. Introduction •Acid Base Balance is a physiological and biochemical mechanism associated to body/blood pH.
- 4. What Is pH? • pH is a Hydrogen ion concentration. • pH = - log [H+] • Different compartment of human body has specific pH. • pH has role in Enzyme activity.
- 5. Why blood pH is Altered?
- 6. •Addition of various acids or alkalies by metabolic activities alters body/blood pH.
- 7. Sources and Types of Acids and Alkalies Added During Metabolic Life Processes
- 8. 8 •Acids are H+ donors. •Bases are H+ acceptors, or give up OH- in solution.
- 10. Acids and Bases can be strong or weak: • A strong acid or base is one that dissociates completely in a solution - HCl, NaOH, and H2SO4 • A weak acid or base is one that dissociates partially in a solution -H2CO3, C3H6O3, and CH2O, Lactate.
- 11. • Acidic Substances of body: –Carbonic acid(H2CO3) –Phosphoric acid( H3PO4) –Sulphuric acid (H2SO4) • Organic Acids: –Lactate, Acetoactate, Pyruvate • Alkaline Substances of body: –Citrate –Bicarbonates.
- 12. What is Acid Base Balance?
- 14. • Acid Base balance is a homeostatic mechanism • Carried out to regulate the altered pH of blood and other body compartments to its normal constant range.
- 15. •Maintenance of Acid Base balance •Is a prime requisite to maintain normal healthy and active life.
- 16. Acid-Base Balance • It is the regulation of HYDROGEN ions. (The more Hydrogen ions, the more acidic the solution and the LOWER the pH) – The acidity or alkalinity of a solution is measured as pH
- 17. Acid Base Balance Regulates pH Why it is Very Essential To Regulate pH?
- 18. • pH of blood and other body compartments are precisely regulated. • pH is always tried to be maintained to its normal constant range.
- 19. •Acid Base Balance maintains the blood pH at normal constant narrow range of 7.35-7.45.
- 20. • pH of the medium directly affects the enzyme activities • Optimum pH is an essential requisite for enzyme activities and normal metabolism.
- 21. •It is prerequisite for regulating blood/body pH: •To maintain normal/optimal Enzyme activities •Normal metabolism •Normal Coordination •Normal Health
- 22. 22 Factors Regulating Acid Base Balance
- 23. Acid Base Balance is Regulated By • First Line of Defense Blood Buffer System • Second Line of Defense –Respiratory Mechanism • Third Line of Defense Renal Mechanism
- 24. 24 1) Chemical Buffers • React very rapidly (less than a second) 2) Respiratory Regulation • Reacts rapidly (seconds to minutes) 3) Renal Regulation • Reacts slowly (minutes to hours)
- 25. Role of Blood Buffer System • First line of defense in mechanism of Acid Base Balance. • Acids (H+) added are neutralized by the salt part of buffer.
- 26. Extracellular Buffers • Bicarbonate Buffer –NaHCO3/H2CO3 (20:1 at 7.4 pH) • Phosphate Buffer –Na2HPO4/NaH2PO4 (4:1 at 7.4 pH) • Protein Buffer –Na-Protein/H-Protein
- 27. Intracellular Buffers • Bicarbonate Buffer –KHCO3/H2CO3 • Phosphate Buffer –K2HPO4/KH2PO4 • Protein Buffer –K-Hb/H-Protein
- 28. Mechanism Action of Buffer Systems • Buffers mixture of weak acids and its salts • Resist change in pH of blood when small amount of acids or alkalis added to the medium.
- 29. •Buffers act quickly but not permanently
- 30. 30 Bicarbonate Buffer System Respiratory Buffer System • Acid - Base balance is primarily concerned with Bicarbonate Buffer mechanism : – H2CO3/ Hydrogen (H+) – Bicarbonate (HCO3 - ) (Alkali Reserve) H+ HCO3 -
- 31. Bicarbonate Buffer • Bicarbonate Buffer- Chief Buffer system of Blood. • NaHCO3 the salt part of buffer neutralizes the strong and non volatile acids added to blood. • It constitutes Alkali reserve(HCO3-)
- 33. 33 Bicarbonate Buffer • Sodium Bicarbonate (NaHCO3) and carbonic acid (H2CO3) • Maintain a 20:1 ratio : HCO3 - : H2CO3 HCl + NaHCO3 ↔ H2CO3 + NaCl NaOH + H2CO3 ↔ NaHCO3 + H2O
- 34. •Action of Bicarbonate (NaHCO3) converts strong dissociable acid into weak non dissociable acid (H2CO3) and a neutral salt without altering the pH.
- 35. • Weak acid H2CO3 formed during buffering action of Bicarbonate buffer is then expired out by Lungs. • Thus Bicarbonate buffer is connected to the respiratory system • Bicarbonate buffer is also termed as Respiratory buffer.
- 36. • Alkali reserve is represented by the concentration of NaHCO3 in the blood. • Alkali reserve concentration(HCO3-) determines the strength of buffering action towards added H+ ions by acids. • More the concentration of Alkali reserve ,more is the buffering action and vice a versa.
- 37. • The blood buffers are effective as long as •The acid load added is not very high and •The alkali reserve (HCO3 -) is not exhausted.
- 38. 38 Phosphate Buffer/Urine Buffer Na2HPO4/NaH2PO4 (4:1 at 7.4 pH) • H+ + HPO4 2- ↔ H2PO4- • OH- + H2PO4 - ↔ H2O + H2PO4 2-
- 39. Phosphate Buffer Mechanism • When H+ ions added they are neutralized/fixed by Na2HPO4 (Alkaline Phosphate) and converted to NaH2PO4 (Acid Phosphates). • These acid phosphates then excreted out through kidneys as acidic urine.
- 40. •Thus Phosphate Buffer is connected to Excretory system . •Phosphate Buffer also termed as Urine Buffer.
- 41. • When an alkali enters it is buffered by the acid phosphate NaH2PO4 which converted to Na2HPO4 alkaline phosphate. • Excreted in urine making it alkaline urine.
- 42. 42 Protein Buffers • Includes hemoglobin, work in blood. • Carboxyl group gives up H+ • Amino Group accepts H+ • The Imidazole group of Histidine present in Hb structure has buffering capacity.
- 43. Role of Respiratory Mechanisms • Respiratory system plays second line of defense mechanism of Acid Base Balance. • Role of respiration in acid base balance is short term regulatory process.
- 44. • H2CO3 formed from Bicarbonate Buffer, is exhaled out through respiratory system. • Increased H2CO3 stimulates the respiratory centre in Medulla Oblongata. • This in turn stimulates hyperventilation which promptly removes H2CO3 from blood by expiration.
- 45. • Exhalation of H2CO3 is as carbon dioxide by activity of enzyme Carbonic Anhydrase of Lungs. • H+ + HCO3 - ↔ H2CO3 ↔ CO2 + H20
- 46. • Respiratory mechanism is powerful, but only works with volatile acids. • Doesn’t affect fixed acids like lactic acid.
- 47. •Blood pH can be adjusted through respiratory mechanism •By changing rate and depth of breathing.
- 48. • Low H2CO3 concentration in blood depresses respiratory centre ,causes hypoventilation i.e slow and shallow respiration. • This retains H2CO3 in blood.
- 49. •If Nervous centre / Respiratory system fails. •Acid Base Balance fails.
- 50. 50 Generation of bicarbonate by RBC LACK OF AEROBIC ACTIVITY,DIFFUSION OF CARBONDIOXIDE,H+ BUFFERED BY HHb.
- 51. 51 Events in lungs and tissue HCO3 - HCO3 - H2CO3 CO2 H2O EXPIRED AIR METABOLISM HHb HHb HbO2 HbO2 H+ H+ O2 O2 CO2 H2O H2CO3 lung tissue Isohydric transport of co2
- 52. 52 Role of Renal Mechanism • Renal mechanism is the third line of defense mechanism. • Role of renal mechanism is long term regulatory process.
- 53. • The acid and alkaline phosphates formed during phosphate buffering mechanism are filtered from blood and excreted out through urine. • Thus the phosphate buffer system is directly connected to renal mechanism.
- 54. • Renal mechanism conserve and produce Bicarbonate ions ( Alkali reserve). • Renal Mechanism is the most effective regulator of blood pH. • If kidneys fail, pH balance fails.
- 55. • Renal System maintains Acid Base Balance through: –Reabsorption of Bicarbonate (HCO3-) ions. –Excretion of H ions –Excretion of titrable acids(Acid Phosphates) –Excretion of Ammonium ions (Glutaminase activity)
- 56. 56 REABSORPTION OF BICARBONATE ~Conservation of Bicarbonate ~Urine is free of HCO3 - ~Simultaneous excretion of H+
- 57. 57 EXCRETION OF TITRABLE ACIDS ~measure of acid excreated by kidney ~no. of millilitres of N/10 NaOH required to titrate 1 litre of urine to pH 7.4 ~role of phosphate buffer
- 58. 58 Excretion Of H+ ions ~Elimination of nonvolatile acid ~Excretion of H+ ~Occurs in PCT ~Regeneration of bicarbonate ~H+ combine with non carbonate base and excreated
- 59. 59 EXCRETION OF AMMONIUM ION NH3 is obtained from Deamination of Glutamine NH4 + cant diffuse back 2/3 of body acid load liberated in the form of NH4 +
- 60. 60 Rates of correction • Buffers function almost instantaneously • Respiratory mechanisms take several minutes to hours • Renal mechanisms may take several hours to days
- 62. 62
- 63. 63
- 64. MECHANISM FOR REGULATION OF ACID BASE BALANCE • Buffer system: temporary solution • Respiratory mechanism provide short time regulation • Renal mechanism : permanent solution • Urine pH < plasma pH ,4.5-9.5 • Eliminate nonvolatile acid, buffered by cation (principally Na+) • Maintain alkali reserve
- 65. Acid Base Imbalance OR Conditions Of Acid Base Disturbances
- 66. 66 The Body and pH • Homeostasis of blood pH is tightly controlled by mechanisms of Acid Base Balance. • Extracellular fluid = 7.4 • Blood pH regulated to = 7.35 – 7.45
- 67. Occurrence of Acid Base Imbalance • When Factors involved in homeostatic mechanisms to regulate Acid Base Balance fails to work efficiently. • Does not maintain the altered pH of blood to normal constant range. • Results into Acid Base Imbalance.
- 68. ACIDOSIS / ALKALOSIS •Two major disturbances in Acid-Base balance –Acidosis –Alkalosis
- 69. Conditions Of Acid Base Imbalance • Acidosis /Acidemia ( Decreased pH/Increased H+ ions) • Alkalosis/Alkalemia (Increased pH/Decreased H+ ions)
- 70. • Acidosis (Acidemia) below 7.35 • Alkalosis (Alkalemia) above 7.45 • Blood pH < 6.8 or > 8.0 death occurs
- 71. 71 ACIDOSIS / ALKALOSIS • Acidosis – A condition in which the blood has too much acid (or too little base), frequently resulting in a decrease in blood pH. • Alkalosis – A condition in which the blood has too much base (or too little acid), occasionally resulting in an increase in blood pH.
- 72. 72
- 74. 74
- 75. Effect of Altered pH •Altered pH may seriously disturbs the vital processes. •Might lead to fatality.
- 76. • Most enzymes function only with narrow pH ranges. • Extremes of pH affects the enzymatic action by protonation or deprotonation at the active sites of Enzymes. • Makes Enzymes inactive.
- 77. • Inactivated Enzymes affect metabolic reactions and metabolic pathways. • Metabolism gets deranged . • Leads to metabolic syndromes.
- 78. 78 pH also affect excitability of Nerve and Muscle cells pH pH Excitability Excitability
- 79. 79 ACID-BASE REGULATION • Enzymes, Hormones and ion distribution are all affected by Hydrogen ion concentrations
- 80. 80 ACIDOSIS / ALKALOSIS • pH changes have dramatic effects on normal cell function 1) Changes in excitability of nerve and muscle cells 2) Influences Enzyme activity 3) Influences K+ levels/Retention of K+
- 81. CHANGES IN CELL EXCITABILITY • pH decrease (more acidic) depresses the central nervous system –Can lead to loss of consciousness • pH increase (more basic)causes over excitability of nervous system. –Tingling sensations, nervousness, muscle twitches
- 82. 82 INFLUENCES ON ENZYME ACTIVITY • pH increases or decreases can alter the shape of the enzyme rendering it non-functional • Changes in enzyme structure can result in accelerated or depressed metabolic actions within the cell
- 83. 83 INFLUENCES ON K+ LEVELS • If H+ concentrations are high (acidosis) than H+ is secreted in greater amounts • This leaves less K+ than usual excreted. • The resultant K+ retention can affect cardiac function and other systems K+ K+ K+ Na+ Na+ Na+ Na+ Na+ Na+ H+ H+ H+ H+ H+ H+ H+ K+ K+ K+ K+ K+
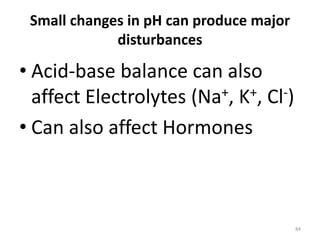
- 84. 84 Small changes in pH can produce major disturbances • Acid-base balance can also affect Electrolytes (Na+, K+, Cl-) • Can also affect Hormones
- 85. ACID-BASE IMBALANCE • Derangements of • Hydrogen/Carbonic acid (H+/H2CO3) • Bicarbonate (HCO3-) concentrations In body fluids are common in conditions of Acid Base Imbalance
- 86. Acid-Base Imbalances •pH< 7.35 Acidosis •pH > 7.45 Alkalosis
- 87. 87 4 Types of Primary Acid-Base Disorders Acid Base Imbalances Biochemical Change Respiratory Acidosis Increased H2CO3 Respiratory Alkalosis Decreased H2CO3 Metabolic Acidosis Metabolic Alkalosis 2 PCO 3 HCO 3 HCO 2 PCO
- 91. Respiratory Acidosis • Primary Carbonic acid excess • Increased H2CO3/Increased pCO2 • Defect in respiratory centre of brain • Defect in respiratory organ system • Decreased elimination of H2CO3 by the lungs. • Hypoventilation
- 92. 92 •Increased blood levels of CO2 above 45 mm Hg. •Hypercapnia – high levels of pCO2 in blood
- 93. 93 RESPIRATORY ACIDOSIS • Respiratory acidosis develops when the lungs don't expel CO2 adequately. • This can happen in diseases that severely affect the lungs.
- 94. • Chronic conditions: – Depression of respiratory center in brain that controls breathing rate – drugs or head trauma – Paralysis of respiratory or chest muscles – Emphysema – Asthma – Pneumonia – Pulmonary edema – Obstruction of respiratory tract – Congestive Cardiac Failure
- 95. HYPOVENTILATION Causes Respiratory Acidosis • Hypo = “Under” Elimination of CO2 H+ pH
- 96. 96 RESPIRATORY ACIDOSIS -breathing is suppressed holding CO2 in body -pH = 7.1 2 20 : CO2 CO2 CO2 CO2
- 97. 97 RESPIRATORY ACIDOSIS • 1) Obstruction of air passages –Vomit, Anaphylaxis, Tracheal Cancer
- 98. 98 RESPIRATORY ACIDOSIS • 2) Decreased Respiration – Shallow, slow breathing – Depression of the respiratory centers in the brain which control breathing rates •Drug overdose
- 99. 99 RESPIRATORY ACIDOSIS • 4) Collapse of lung –Compression injury, open thoracic wound Left lung collapsed
- 100. 100 Respiratory Acidosis • Acute conditions: –Adult Respiratory Distress Syndrome –Pulmonary edema –Pneumothorax
- 101. 101 Compensation for Respiratory Acidosis •Kidneys eliminate hydrogen ion and retain bicarbonate ions.
- 102. 102 Signs and Symptoms of Respiratory Acidosis • Breathlessness • Restlessness • Lethargy and disorientation • Tremors, convulsions, coma • Respiratory rate rapid, then gradually depressed • Skin warm and flushed due to vasodilation caused by excess CO2
- 103. 103 Treatment of Respiratory Acidosis •Restore ventilation •IV lactate solution •Treat underlying dysfunction or disease
- 105. 105 Respiratory Alkalosis • Primary Carbonic acid deficit • Decreased H2CO3 • pCO2 less than 35 mm Hg (hypocapnea) • Most common acid-base imbalance • Primary cause is hyperventilation • Washes out excessive quantity of H2CO3 through expiration process of lungs.
- 106. • Stimulation of respiratory centre in brain • Hyperventilation
- 107. 107 Respiratory Alkalosis • Conditions that stimulate respiratory center: – Oxygen deficiency at high altitudes – Pulmonary disease and Congestive heart failure – caused by hypoxia – Respiratory center lesions – Acute anxiety – Fever, anemia – Early salicylate intoxication – Cirrhosis – Gram-negative sepsis/Meningitis
- 108. 108 RESPIRATORY ALKALOSIS • Anxiety is an emotional disturbance • The most common cause of hyperventilation, and thus respiratory alkalosis, is noted in anxiety
- 109. 109 RESPIRATORY ALKALOSIS • Respiratory center lesions –Damage to brain centers responsible for monitoring breathing rates • Tumors • Strokes
- 110. 110 RESPIRATORY ALKALOSIS • High Altitude – Low concentrations of O2 in the arterial blood reflexly stimulates ventilation in an attempt to obtain more O2 – Too much CO2 is “blown off” in the process
- 111. 111 RESPIRATORY ALKALOSIS • Fever –Rapid shallow breathing blows off too much CO2
- 112. 112 RESPIRATORY ALKALOSIS • Salicylate poisoning (Aspirin overdose) –Ventilation is stimulated without regard to the status of O2, CO2 or H+ in the body fluids
- 113. 113 RESPIRATORY ALKALOSIS • Kidneys compensate by: –Retaining hydrogen ions –Increasing bicarbonate excretion H+ HCO3 - HCO3 - HCO3 - HCO3 - HCO3 - HCO3 - HCO3 - HCO3 - HCO3 - HCO3 - H+ H+ H+ H+ H+ H+ H+ H+ H+ H+
- 114. 114 HYPERVENTILATION Causes Respiratory Alkalosis • Hyper = “Over” Elimination of CO2 H+ pH
- 115. 115 Compensation of Respiratory Alkalosis • If kidneys are functioning normal • The conditions of respiratory acidosis or alkalosis are compensated. • Kidneys conserve hydrogen ion • Excrete bicarbonate ion
- 116. 116 Treatment of Respiratory Alkalosis • Treat underlying cause • Breathe into a paper bag • IV Chloride containing solution Cl- ions replace lost bicarbonate ions
- 118. 118 Metabolic Acidosis • Primary Alkali deficit • Bicarbonate deficit - blood concentrations of bicarbonate drop below 22mEq/L • Causes: – Loss of bicarbonate through diarrhea or renal dysfunction. – Overproduction production of acids (lactic acid or ketones) – Failure of kidneys to excrete H+
- 119. 119 METABOLIC ACIDOSIS • Occurs when there is a decrease in the normal 20:1 ratio –Decrease in blood pH and bicarbonate level • Excessive H+ or decreased HCO3 - H2CO3 HCO3 - 1 20 : = 7.4 1 10 : = 7.4
- 120. 120 METABOLIC ACIDOSIS • Any acid-base imbalance not attributable to CO2 is classified as metabolic –Metabolic production of Acids –Or loss of Bases
- 121. 121 METABOLIC ACIDOSIS • The causes of metabolic acidosis can be grouped into five major categories –1) Ingesting an acid or a substance that is metabolized to acid –2) Abnormal Metabolism –3) Kidney Insufficiencies –4) Strenuous Exercise –5) Severe Diarrhea
- 122. 122 METABOLIC ACIDOSIS • 1) Ingesting An Acid – Most substances that cause acidosis when ingested are considered poisonous – Examples include wood alcohol (methanol) and antifreeze (ethylene glycol) – However, even an overdose of aspirin (acetylsalicylic acid) can cause metabolic acidosis
- 123. METABOLIC ACIDOSIS • 2) Abnormal Metabolism – The body can produce excess acid as a result of several diseases • Ketoacidosis • Type I Diabetes Mellitus • Uncontrolled Diabetes mellitus • Prolonged Starvation • Lacticacidosis • Shock • Haemorrhage • Violent Exercise-
- 124. 124 METABOLIC ACIDOSIS • Unregulated diabetes mellitus causes ketoacidosis –Body metabolizes fat rather than glucose –Accumulations of metabolic acids (Keto Acids) cause an increase in plasma H+
- 125. METABOLIC ACIDOSIS • 3) Kidney Insufficiencies –This type of kidney malfunction is called renal tubular acidosis or uremic acidosis and may occur in people with kidney failure or with abnormalities that affect the kidneys' ability to excrete acid
- 126. 126 METABOLIC ACIDOSIS • 3) Kidney Insufficiencies –Kidneys may be unable to rid the plasma of even the normal amounts of H+ generated from metabolic acids –Kidneys may be also unable to conserve an adequate amount of HCO3 - to buffer the normal acid load
- 127. 127 METABOLIC ACIDOSIS • 4) Strenuous Exercise –Muscles resort to anaerobic glycolysis during strenuous exercise –Anaerobic respiration leads to the production of large amounts of lactic acid C6H12O6 2C3H6O3 + ATP (energy) Enzymes Lactic Acid
- 128. METABOLIC ACIDOSIS • 5) Severe Diarrhea – Fluids rich in HCO3 - are released and reabsorbed during the digestive process – During diarrhea this HCO3 - is lost from the body rather than reabsorbed
- 129. METABOLIC ACIDOSIS • 5) Severe Diarrhea – The loss of HCO3 - without a corresponding loss of H+ lowers the pH – Less HCO3 - is available for buffering H+ – Prolonged deep (from duodenum) vomiting can result in the same situation
- 130. 130 Symptoms of Metabolic Acidosis •Headache, lethargy •Nausea, vomiting, diarrhea •Coma •Death
- 131. 131 Compensation for Metabolic Acidosis • Increased ventilation. • Renal excretion of hydrogen ions if possible. • K+ exchanges with excess H+ in ECF. • H+ into cells, K+ out of cells.
- 132. 132 Treatment of Metabolic Acidosis • IV lactate solution
- 134. 134 Metabolic Alkalosis • Bicarbonate Excess - concentration in blood is greater than 26 mEq/L • Causes: – Excess vomiting = loss of stomach acid – Excessive use of alkaline drugs – Certain diuretics – Endocrine disorders – Heavy ingestion of antacids – Severe dehydration – Cushings Syndrome – Prolonged exposure to x rays and UV rays
- 135. 135 METABOLIC ALKALOSIS • Elevation of pH due to an increased 20:1 ratio –May be caused by: • An increase of bicarbonate • A decrease in hydrogen ions –Imbalance again cannot be due to CO2 –Increase in pH which has a non-respiratory origin 7.4
- 136. 136 METABOLIC ALKALOSIS • Can be the result of: –1) Ingestion of Alkaline Substances –2) Vomiting ( loss of HCl )
- 137. 137 METABOLIC ALKALOSIS • Baking soda (NaHCO3) often used as a remedy for gastric hyperacidity –NaHCO3 dissociates to Na+ and HCO3 -
- 138. 138 Compensation for Metabolic Alkalosis • Alkalosis most commonly occurs with renal dysfunction, so can’t count on kidneys. • Respiratory compensation difficult – hypoventilation limited by hypoxia.
- 139. 139 Symptoms of Metabolic Alkalosis • Respiration slow and shallow • Hyperactive reflexes ; tetany • Often related to depletion of electrolytes • Atrial tachycardia • Dysrhythmias
- 140. 140 Treatment of Metabolic Alkalosis •Electrolytes to replace those lost •IV chloride containing solution •Treat underlying disorder
- 141. 141 Acidosis • Principal effect of acidosis is depression of the CNS through ↓ in synaptic transmission. • Generalized weakness • Deranged CNS function the greatest threat • Severe acidosis causes – Disorientation – Coma – Death
- 142. 142 Alkalosis • Alkalosis causes over excitability of the central and peripheral nervous systems. • Numbness • Light headedness • Severe Alkalosis causes : – Nervousness – muscle spasms or Tetany – Convulsions – Loss of consciousness – Death
- 143. Compensation Of Acid Base Imbalance • The body response to acid-base imbalance is called compensation • May be complete compensation if altered pH brought back within normal limits • Partial compensation if pH range is still outside norms. • Uncompensated if pH range is very out from norms.
- 144. • If underlying problem is respiratory, renal mechanisms can bring about metabolic compensation. • If underlying problem is metabolic, hyperventilation or hypoventilation can help : respiratory compensation.
- 145. 145 ACIDOSIS decreased removal of CO2 from lungs failure of kidneys to excrete acids metabolic acid production of keto acids absorption of metabolic acids from GI tract prolonged diarrhea accumulation of CO2 in blood accumulation of acid in blood excessive loss of NaHCO3 from blood metabolic acidosis deep vomiting from GI tract kidney disease (uremia) increase in plasma H+ concentration depression of nervous system accumulation of CO2 in blood accumulation of acid in blood excessive loss of NaHCO3 from blood respiratory acidosis
- 146. 146 ALKALOSIS respiratory alkalosis anxiety overdose of certain drugs high altitudes prolonged vomiting ingestion of excessive alkaline drugs excess aldosterone hyperventilation loss of CO2 and H2CO2 from blood loss of acid accumulation of base metabolic alkalosis decrease in plasma H+ concentration overexcitability of nervous system hyperventilation loss of CO2 and H2CO2 from blood loss of acid accumulation of base
- 147. Organ dysfunction And Acid Base Imbalance • CNS – respiratory acidosis (suppression) and alkalosis (stimulation) • Pulmonary – respiratory acidosis (COPD) and alkalosis (hypoxia, pulmonary embolism) • Cardiac – respiratory alkalosis, respiratory acidosis, metabolic acidosis (pulmonary edema) • GIT – metabolic alkalosis (vomiting) and acidosis (diarrhea) • Liver – respiratory alkalosis, metabolic acidosis (liver failure) • Kidney – metabolic acidosis (RTA) and alkalosis (1st Aldosterone)
- 148. Organ Dysfunction • Endocrine – Diabetes mellitus – metabolic acidosis – Addisons Disease/Adrenal insufficiency – metabolic acidosis.(Decreased H+ ions excretion) – Cushing’s Syndrome – metabolic alkalosis (Increased Cortisol- Increased H+ ions excretion) – Primary aldosteronism – metabolic alkalosis • Drugs/toxins – Toxic alcohols – metabolic acidosis – ASA/Aspirin – metabolic acidosis and respiratory alkalosis( Causes Hyperventilation) – Theophylline overdose – respiratory alkalosis
- 149. 149 ACID – BASE DISORDERS Clinical State Acid-Base Disorder Pulmonary Embolus Respiratory Alkalosis Cirrhosis Respiratory Alkalosis Pregnancy Respiratory Alkalosis Diuretic Use Metabolic Alkalosis Vomiting Metabolic Alkalosis Chronic Obstructive Pulmonary Disease Respiratory Acidosis Shock Metabolic Acidosis Severe Diarrhea Metabolic Acidosis Renal Failure Metabolic Acidosis Sepsis (Bloodstream Infection) Respiratory Alkalosis, Metabolic Acidosis
- 150. 150
- 151. Anion Gap
- 152. • Sum of anion and cations is always equal • Sodium and Potassium accounts for 95% of cations • Chloride and bicarbonate accounts for 68% of anions • There is difference between measured anion and cation • The unmeasured anions constitute the ANION GAP.
- 153. • They are protein anions ,sulphates ,phosphates and organic acid(Unmeasured Anions) • AG can be calculated as (Na+ + K+)—(HCO3 - + Cl-) • High anion gap acidosis: renal failure, DM • Normal anion gap acidosis: diarrhea • Hyperchloremic acidosis
- 154. Calculation Of Anion Gap • Na ++ K+ = Cl- + HCO3 - + A- • 136+ 4 = 100 + 25 • A- = 15 mEq/L
- 155. • Normal AG is typically 12 ± 4 mEq/L. • If AG is calculated using K+, the normal AG is 16 ± 4 mEq/L
- 156. Significance of Anion Gap Calculation •Calculation of Anion gap and its values help in diagnosing conditions of Acid Base Balance and Imbalance.
- 157. • The anion gap is increased in conditions such as metabolic acidosis: • That result from elevated levels of metabolic acids (metabolic acidosis) –Lactic acidosis –Diabetic Ketoacidosis –Renal Failure
- 158. • A low anion gap occurs in conditions that cause a fall in unmeasured anions • (primarily albumin) OR a rise in unmeasured cations
- 160. Calculate the Anion Gap • 1. Calculate the anion gap as described. • 2. An anion gap ,over 25 suggests a severe metabolic acidosis. • 3. Causes of an high anion gap: ethylene glycol, lactic acid, methanol, paraldehyde, aspirin, renal failure, ketoacidosis (diabetic or ethanol).
- 161. Anion Gap Acidosis: • Anion gap >12 mmol/L; caused by a decrease in [HCO3 -] • Balanced by an increase in an unmeasured acid ion from either endogenous production or exogenous ingestion (normochloremic acidosis).
- 162. 1. Normal gap 2. Increased gap 1. Renal “HCO3” losses 2. GI “HCO3” losses Proximal RTA Distal RTA Diarrhea 1. Acid prod 2. Acid elimination Lactate DKA Ketosis Toxins Alcohols Salicylates Iron Renal disease Metabolic Acidosis and the Anion gap
- 164. Henderson Hasselbalch Equation • pH= pka +log [HCO3-]/[H2CO3] • At pH 7.4 the ratio of HCO3-/H2CO3 is 1:20. • A buffer is most effective when pH=pKa • When concentration of salt and acid are equal.
- 165. Significance of Henderson Hasselbalch Equation •The equation helps in calculating pH of Buffers. •The equation helps in assessing status of Acid Base balance.
- 166. Stepwise Approaches • History & physical examination • Arterial blood gas for pH, pCO2, (HCO3) – Use the HCO3 from ABG to determine compensation • Serum Na, K, Cl, CO2 content – Use CO2 content to calculate anion gap • Calculate anion gap – Anion gap = {Na - (Cl + CO2 content)} • Determine appropriate compensation • Determine the primary cause
- 168. • Arterial Blood Gas(ABG )Analyzer determines Acid Base Balance and Imbalance.
- 169. 169 Diagnosis of Acid-Base Imbalances 1. Note whether the pH is low (acidosis) or high (alkalosis) 2. Decide which value, pCO2 or HCO3 - , is outside the normal range 3. If the cause is a change in pCO2,/H2CO3 the problem is respiratory. 4. If the change is in HCO3 - the problem is metabolic.
- 170. Normal Arterial Blood Gas (ABG) Lab Values: • Arterial pH: 7.35 – 7.45 • HCO3 -: 22 – 26 mEq/L • PCO2: 35 – 45 mmHg • TCO2: 23 – 27 mmol/L • PO2: 80 – 100 mmHg • Base Excess: -2 to +2 • Anion Gap: 12 – 14 mEq/L
- 171. 171 Example • A patient is in intensive care because he suffered a severe myocardial infarction 3 days ago. The lab reports the following values from an arterial blood sample: – pH 7.3 – HCO3- = 20 mEq / L ( 22 - 26) – pCO2 = 32 mm Hg (35 - 45)
- 172. 172 Diagnosis • Metabolic acidosis • With compensation
- 173. Questions • Long Essays. • What is acid-base balance? Describe the homeostatic mechanism by which the blood pH is regulated. • Short Notes • Blood Buffer System. • Role of Kidney in acid-base balance. • Hb as Buffer system. • Acid-Base imbalance. • Metabolic Acidosis. • Difference between acidosis & alkalosis. • Anion Gap.
- 174. 174 END ACID - BASE BALANCE THANKS
- 175. THANK YOU



![What Is pH?
• pH is a Hydrogen ion concentration.
• pH = - log [H+]
• Different compartment of human
body has specific pH.
• pH has role in Enzyme activity.](https://image.slidesharecdn.com/finalacidbasebalane-221129072011-7cf47179/85/final_acid_base_balane-4-320.jpg)



























































































































































![Anion Gap Acidosis:
• Anion gap >12 mmol/L; caused by a decrease
in [HCO3 -]
• Balanced by an increase in an unmeasured
acid ion from either endogenous production
or exogenous ingestion (normochloremic
acidosis).](https://image.slidesharecdn.com/finalacidbasebalane-221129072011-7cf47179/85/final_acid_base_balane-161-320.jpg)


![Henderson Hasselbalch Equation
• pH= pka +log [HCO3-]/[H2CO3]
• At pH 7.4 the ratio of HCO3-/H2CO3
is 1:20.
• A buffer is most effective when
pH=pKa
• When concentration of salt and acid
are equal.](https://image.slidesharecdn.com/finalacidbasebalane-221129072011-7cf47179/85/final_acid_base_balane-164-320.jpg)










