Next Generation Sequencing
- 1. Next Generation Sequencing By: Sajad Rafatiyan Faculty of Advanced Sciences and Technologies University of Isfahan
- 2. Contents •Introduction •NGS Tools •NGS Machines •Different methods of NGS •Sequencing by Ligation •Illumina sequencing •454 sequencing •Ion Torrent: Proton / PGM sequencing •Single Molecule Real-Time Sequencing (SMRT) •GradION Nanopore Sequancing •Sources
- 3. Introduction In 1953 James Watson and Francis Crick described the double helix of DNA. In 1977 Frederick Sanger invented a method for sequencing the DNA sequence using dideoxynucleosides. Through this method and via using fluorescence tagged nucleotides and chromatography, sequencing 600-800 bp sequences is possible.
- 4. In 1960 Thomas Dale Brock reported hyperthermophiles living in hot springs at Yellowstone National Park. After that in 1976 Chien et al isolated thermostable DNA polymerase form Thermus aquaticus, and now we use it for PCR. PCR (Polymerase Chain Reaction) was Develop in 1983 by Kary Mullis.
- 5. In 1990 Illumina/solax, Roch/454 Life Science and ABI/SoliD made some machines for DNA sequencing separately. The machines have been commercialy available in 2005-2006. Bioinformatic tools can be used for sequence analysis and the whole genome of organism is sequenced.
- 6. NGS Tools Visualization Integrative Genomics Viewer (IGV) Artemis Abrowse Integrated Genome Browser (IGB)
- 7. Atfirstitwasreally expansive to sequencinga DNAmolecule, efficiency wasnotreally good andit took long time tosequencingamolecule. Today Illumina has been insisted which can sequencing human genome in one hour by 100 dollars
- 8. NGS Machines
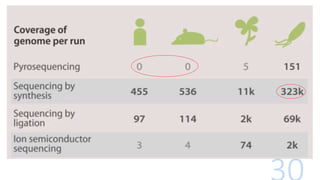
- 11. Different methods of sequencing 1. Pyrosequencing 2. Sequencing by Synthesis 3. Sequencing by Ligation 4. Ion Semiconductor Sequencing
- 15. Illumina sequencing In NGS, vast numbers of short reads are sequenced in a single stroke. To do this, firstly the input sample must be cleaved into short sections. The length of these sections will depend on the particular sequencing machinery used. In Illumina sequencing, 100-150bp reads are used. Somewhat longer fragments are ligated to generic adaptors and annealed to a slide using the adaptors. PCR is carried out to amplify each read, creating a spot with many copies of the same read. They are then separated into single strands to be sequenced.
- 16. The slide is flooded with nucleotides and DNA polymerase. These nucleotides are fluorescently labelled, with the color corresponding to the base. They also have a terminator, so that only one base is added at a time.
- 17. An image is taken of the slide. In each read location, there will be a fluorescent signal indicating the base that has been added.
- 18. Theslide isthenpreparedforthe next cycle.The terminators are removed, allowing thenextbaseto be added,andthe fluorescent signal is removed, preventingthe signal from contaminatingthenext image. Theprocess isrepeated, addingone nucleotideata time andimaging inbetween.
- 19. Computersare thenused to detectthebaseateachsite in eachimage andthese are usedto constructa sequence. All of thesequence readswill be the same length,as thereadlength dependson thenumberof cyclescarriedout.
- 20. 454 sequencing Roche 454 sequencing can sequence much longer reads than Illumina. Like Illumina, it does this by sequencing multiple reads at once by reading optical signals as bases are added. As in Illumina, the DNA or RNA is fragmented into shorter reads, in this case up to 1kb. Generic adaptors are added to the ends and these are annealed to beads, one DNA fragment per bead. The fragments are then amplified by PCR using adaptor-specifc primers. Each bead is then placed in a single well of a slide. So each well will contain a single bead, covered in many PCR copies of a single sequence. The wells also contain DNA polymerase and sequencing buffers.
- 21. The slide is flooded with one of the four NTP species. Where this nucleotide is next in the sequence, it isadded to the sequence read. If that single base repeats, then more will be added. So if we flood with Guanine bases, and the next in a sequence is G, one G will be added, however if the next part of the sequence is GGGG, then four Gs will beadded.
- 22. The addition of each nucleotide releases a light signal. These locations of signals are detected and used to determine which beads the nucleotides are added to.
- 23. This NTPmix is washedaway.The next NTPmix is now addedandthe processrepeated, cyclingthroughthefourNTPs.
- 24. This kind of sequencing generates graphs for each sequence read, showing the signal density for each nucleotide wash. The sequence can then be determined computationally from the signal density in each wash. All of the sequence reads we get from 454 will be different lengths, because different numbers of bases will be added with each cycle.
- 25. Ion Torrent: Proton / PGM sequencing Unlike Illumina and 454, Ion torrent and Ion proton sequencing do not make use of optical signals. Instead, they exploit the fact that addition of a dNTP to a DNA polymer releases an H+ ion. As in other kinds of NGS, the input DNA or RNA is fragmented, this time ~200bp. Adaptors are added and one molecule is placed onto a bead. The molecules are amplified on the bead by emulsion PCR. Each bead is placed into a single well of a slide.
- 26. Like 454, the slide is flooded with a single species of dNTP, along with buffers and polymerase, one NTP at a time. The pH is detected is each of the wells, as each H+ ion released will decrease the pH. The changes in pH allow us to determine if that base, and how many thereof, was added to the sequence read.
- 27. ThedNTPsarewashedaway,and theprocessis repeated cycling throughthedifferent dNTPspecies.
- 28. ThepH change,if any,is used to determine howmanybases (if any) were addedwitheach cycle.
- 31. SMRT (Single Molecule Real-Time sequencing)
- 32. SMRT Sequencing
- 33. Advantages SMRT Sequencing •Deconvolute complex mixtures of unique haplotypes •Accurately identify somatic variants •Resolve complex communities
- 35. GridION Nanopore
- 37. Application of NGS •Whole Genome Sequencing (to find point mutation or be sure about gene integration in right place) •Target Sequencing (hotspot sequences mutation for cancer or immune system disease) •De Novo Sequencing and Assembly (for new organism which have not enough information about them) •RNA-Sequencing (to detect coding and non-coding sequences and sometime we can use it as genome sequence) •Epigenetic changes
- 38. Application of NGS •Single cell sequencing •Free DNA sequencing (detection of cancer or genetic disorders before birth) •Long non-coding RNA interactions (fore gene translation regulation) •For Methylation Assisted Isolation of Regulatory Elements (FAIREDNase sequencing)
- 39. Sources Wong, L.-J. C., 2013. Next Generation Sequencing: Translation to Clinical Diagnostics. 1st ed. NewYork: springer Jay Shendure & Hanlee Ji, 2008. Next-generation DNA sequencing. Nature Biotechnology, 26(10). https://www.genewiz.com/en/Public/Services/Next-Generation-Sequencing https://www.ebi.ac.uk/training/online/course/ebi-next-generation-sequencing- practical-course https://nanoporetech.com/applications/dna-nanopore-sequencing