PHARMACOKINETICS-II presentation meta,ex
- 1. PHARMACOKINETICS-II MENTOR- PRESENTED BY- Prof. Dr . S. Kothari Dr . Asmita Pandey
- 2. PHARMACOKINETICS Drug movement inside body PHARMACOKINETICS also known as ADME studies The study of action of body on the administered drug.
- 3. Biotransformation / Metabolism • Lipophilic compounds though easily absorbed need to become hydrophilic for excretion. Biotransformation is thus the process of conversion of lipophilic compounds to hydrophilic compounds to facilitate their elimination • Lipophilic compounds though get filtered through the renal glomeruli are reabsorbed through the renal tubules into the systemic circulation.
- 4. Outcomes of biotransformation • Active drug to inactive metabolite (most drugs) • Active drug to active metabolites • Inactive drug to active metabolites (prodrug) • Active drug to toxic metabolites.
- 5. Drug Active metabolite Halothane Trifluoroacetic acid Isoniazid Acetyl hydrazine Methoxyflurane Fluoride Paracetamol NAPQI Sulphonamides Acetyl derivatives Active drug Active metabolite Amitriptyline Nortriptyline Carbimazole Methimazole Chloroquine Hydroxychloroquine Codeine Morphine Digitoxin Digoxin Imipramine Desipramine Spironolactone Canrenone
- 6. Prodrug Prodrugs have been developed with the aim of achieving the significant concentration at desired site and also minimising the side effects. • Advantages i. Drug reaches in maximum concentration at the desired site e.g. dopamine (doesn’t cross BBB) while levodopa (crosses BBB) ii. Prolong the duration of action e.g. antipsychotic fluphenazine decanoate iii. Provide site specific delivery e.g. methenamine to formaldehyde in urine at acidic pH to produce local effect
- 7. Disadvantages 1.Cannot be used for emergency situations 2.Cannot be activated to achieve desired therapeutic concentration in liver disease/damage as they are activated in liver.
- 8. Prodrug inactive Active drug metabolite Clorazepate Desmethyl diazepam Enalapril Enalaprilat Levodopa Dopamine Pivempicillin Ampicillin Prednisone Prednisolone Proguanil Cycloguanil Terfenadine Fexofenadine
- 9. Sites of biotransformation Organ Site Drug Liver Most drugs Gastrointestinal tract Tyramine Chloramphenicol Plasma Succinyl choline Kidney Desipramine , morphine Skin Hydrocortisone , vidarabine
- 10. Phase I reactions/ functionalisation reaction Phase II reactions/ conjugation reaction Conversion of nonpolar to polar by unmasking or adding functional group making it polar. Mainly conjugation reactions Degradative/catabolic changes Anabolic/synthetic/conjugative changes Carried out by microsomal enzymes. Carried out by cytoplasmic or mitochondrial enzymes (except Glucuronidation) Ex :- Oxidation, reduction, hydrolysis, dehydrogenation, deamination, cyclisation, de cyclisation, halogenation EX:- glucuronide, acetylation, sulfation, glycation, glucuronide conjugation Not all drug compounds go through this phase of metabolism. Enzymes exhibit polymorphism.
- 11. PHASE-I Reactions Oxidation Microsomal Oxidation CYP 450 dependent 1. Aromatic Hydroxylations- Phenobarbitone to p-Hydroxyphenobarbitone 2. Aliphatic Hydroxylations- Phenobarbitone to Hydroxyphenobarbitone Non-Microsomal Oxidation- CYP45 independent 1. Mitochondrial Oxidation – oxidation of MAO(x) Catecholamines (Epi VMA) 2. Cytoplasmic Oxidation Alc.DH Ald.DH Alcohol Aldehyde Acetic acid
- 12. 3.N-, O-, S- Dealkylation – mephobarbitone to phenobarbitone. 4.N-, and S- Oxidation 5.Deamination – Amphetamine to Phenylacetone 6.Desulfaration – parathion to paraoxon 6-methylthiopurine to mercaptopurine 3. Plasma Oxidative Processes Xanthine oxidase converts Xanthine to uric acid Histaminase converts Histamine to imidazole acetic acid
- 13. Reduction Microsomal Reduction NITRO REDUCTION AZO REDUCTION KETO REDUCTION Chloramphenicol to 1.Prontosil to 1.Cortisone to Hydrocortisone Arylamine metabolite Sulfanilamide 2.reduction of Methadone & Naloxone to hydroxylated 2.Reduction of Sulfasalazine metabolites Non Microsomal Reduction Chloral hydrate Trichlorethanol
- 14. Hydrolysis Microsomal Hydrolysis • Rare • Except i. Pethidine to pethidinic acid (meperidine) ii. hydrolysis of lidocaine by hepatic esterase
- 15. Non-Microsomal Hydrolysis • For esters and amides (esterase and amidases) • Also peptidase, protease and phosphatases. e.g. Hydrolysis of beta lactam ring of penicillin G Procaine to PABA by plasma choline esterase
- 16. • Enzymes which metabolize the Drug • Location- SER of LIVER also, Kidneys, Intestinal mucosa, Lungs. • Non Specific Action • Metabolize ONLY LIPID SOLUBLE drugs. Main enzymes • CYTOCHROME P 450 (CYP450) • MIXED FUNCTION OXIDASES (MFOs) • GLUCORONYL TRANSFERASES MICROSOMAL ENZYMES
- 18. derived from the spectrophotometric peak at the wavelength of the absorption maximum of the enzyme (450 nm) when it is in the reduced state and complexed with carbon monoxide. (HEME- pigment) CYP450 1,2,3…./ A,B,C,D…../1,2,3,4….. FAMILY OF ENZYME SUBFAMILY SPECIFIC ISOENZYME or GENE NUMBER
- 19. Known CYP450 in humans belong to FAMILY 1, 2, and 3 and their respective subfamilies and isoenzymes known are CYP450 1A1,1A2,1B1 ; 2A6,2B1,2B6, 2C8, 2C9, 2C19 ; 2D6, 2E1 ; 3A4, 3A5 Most important for drug metabolism CYP 3A CYP 2D Exhibit polymorphism CYP 2C (1 gene- multiple mRNA coding for enzymes) CYP2E
- 20. CYP Enzymes Substrate CYP1A2 Theophylline CYP2C8/CYP2C9 (MINIMUM) Warfarin , phenytoin CYP2C19 Clopidogrel Voriconazole Ppi CYP2B6 Bupropion CYP2E1 Paracetamol
- 21. CYP2D6 Beta blockers Anti-arrythmias-except amiodarone TCA SSRI Opiods Neuroleptics CYP3A4 (MAXIMUM) Astemizole , amiodarone Benzodiazepines Cisapride Calcinurin inhibitors Estrogen Diltiazem Estrogen Fexofenadine Grape fruit juice Statins
- 22. PHASE-II Reactions Includes all types of Conjugation Reactions Microsomal conjugation GLUCURONIDE CONJUGATION • Only example of this type of conjugation. • Its an exception and is included in Phase I reactions. • Phase I metabolites + UDPGA Polar conjugates which are usually inactive easily excreted out Non-microsomal conjugation Glucuronyl transferase • N- Acetyl Conjugation • Sulphate Conjugation • Amino Acid Conjugation • Methyl Conjugation • Glutathione Conjugation • Ribosides and Riboside Phosphates
- 24. Types of CONJUGATION Co Factors & Enzymes Drugs involved Sulfate conjugation 3’Phosphoadenosine-5- phosphosulfate (PAPS) Sulphotransferase • Aspirin • Methyldopa • PCM • Corticosteroids • Chloramphenicol Amino acid conj. Acyl Co-enzyme A Glycine transferase • Aspirin • Benzoic acid • Nicotinic acid Methyl conj. S-A-Methionine Transmethylase • Dopamine • Epinephrine • Histamine
- 25. Types of CONJUGATION Co Factors & Enzymes Drugs involved N Acetyl Conjugation Acetyl Co-A N acetyl transferase • Aromatic amines • Isoniazid • PAS • Dapsone • Sulfonamides Glutathione Conj. • Epoxides • NO2 groups • Ethacrynic acid • Sulfbromophthalein Ribosides & Riboside Phosphates • Purines and Pyrimidines used as antimetabolites • Form Ribonucleosides and Ribonucleotides
- 26. Enzyme induction • Reversible • Increases the microsomal enzyme activity thus increases the metabolism of the drug. • Clinical Importance- Decreases plasma level Decreases drug therapeutic effect if inactive metabolite is produced and vice versa. E.g. 1. OCP+ Phenytoin/Rifampicin – unwanted pregnancy 2. Barbiturates + Warfarin (high doses) 3. Phenytoin – increases the metabolism Of Vit D3 Osteomalacia 4. Barbiturates – enhance their own metabolism pharmacokinetic tolerance • Drug Toxicity – Alcoholics-- compromised liver ; HEPATOTOXICITY with Paracetamol overdose or even with therapeutic dose Due to N-acetyl p- benzoquiononeimine which is a toxic metabolite of PCM
- 27. Enzyme inhibition • One drug inhibit metabolism of the other drug • Rapid and Reversible process • Irreversible in Secobarbital overdoses (impairs its own metabolism. • E.g. 1. Theophylline + chloramphenicol/erythromycin- cause Nausea and vomiting and tremors. 2. Dicumarol + Cimetidine excessive bleeding 3. Morphine + MAO inhibitor Severe Resp. Depression 4. L-Dopa + Carbidopa more availability of L-Dopa to pass BBB. 5. Aversion to alcohol by Disulfiram nausea, vomiting and headache. 6. Reversal of skeletal muscle paralysis due to D- tubocurare by Neostigmine.
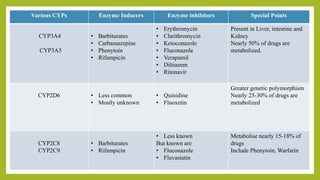
- 28. Various CYPs Enzyme Inducers Enzyme inhibitors Special Points CYP3A4 CYP3A5 • Barbiturates • Carbamazepine • Phenytoin • Rifampicin • Erythromycin • Clarithromycin • Ketoconazole • Fluconazole • Verapamil • Diltiazem • Ritonavir Present in Liver, intestine and Kidney Nearly 50% of drugs are metabolized. CYP2D6 • Less common • Mostly unknown • Quinidine • Fluoxetin Greater genetic polymorphism Nearly 25-30% of drugs are metabolized CYP2C8 CYP2C9 • Barbiturates • Rifampicin • Less known But known are • Fluconazole • Fluvastatin Metabolise nearly 15-18% of drugs Include Phenytoin, Warfarin
- 29. Various CYPs Enzyme Inducers Enzyme inhibitors Special Points CYP2C19 • Barbiturates • Rifampicin --- Metabolise 12-15 drugs like Diazepam, Omeprazole CYP1A1 CYP1A2 • Lot of drugs and pollutants • Barbiturates • Rifampicin • Carbamazepine • SMOKING --- Few drugs metabolised like Theophylline, warfarin, Clomipramine, Paracetamol Cyp1A1 induced in lungs of smokers. CYP2E1 • Chronic alcohol consumption • Paracetamol • Disulfiram Metabolises Few drugs used in Gen.Anaesthesia.
- 30. NOTE:- Water soluble drugs such as- i. Penicillin G ii. Aminoglycosides iii. Heparin Do not require metabolism / biotransformation since these being water soluble get eliminated out of the body without getting metabolised.
- 31. NON-MICROSOMAL ENZYMES Reactions Catalysed by them are all Phase II reactions EXCEPT Glucuronide Conjugation E.g. • MAO • Esterases • Amidases • Transferases • Conjugases Non inducible But can be inhibitory Shows genetic variations. • E.g. Acetyl transferases • Pseudo choline-esterases Location- • Cytoplasm • Mitochondria of hepatic cells • Plasma
- 32. Factors Affecting Drug Metabolism • Age – Younger the age lower the number of microsomal enzyme older the age lower the number of the enzyme. • Nutrition – High protein and Low carbs increase rate of metabolism. • Sex • Species • Race • Genetic Variations
- 33. Once the drug is metabolised it needs to be excreted out of the body in the water soluble form or sometimes even in unchanged form . Kidneys are the main organ for drug elimination/excretion Example of drugs excreted unchanged in urine- Acetazolamide, ampicillin, amiloride, trimethoprim, atenolol, gentamycin etc. Drug elimination/excretion
- 34. Routes of Excretion Major Routes • Renal • Biliary • Faecal • Alveolar Minor Routes • Milk • Skin • Saliva • Sweat • Hair
- 35. RENAL EXCRETION Glomerular Filtration • Molecular Size – <20,000 Dal. Can be filtered. Insulin, Heparin not Filtered • Plasma Protein Binding (PPB) more is the PPB less is the filteration. Eg. WARFARIN • RBF- more is the blood flow more is the filteration. Tubular Secretion Drug Glomerulus PCT Secreted in lumen ( Carrier Mediated Active Transport ) + Protein binding As a result the drug gets bound competitively and helps the other drugs by decreasing the secretion and increasing plasma conc. Tubular Reabsorption • Passive diffusion Transport • Depends on 1. Lipid Solubility 2. Ionisation constant 3. pH of urine Acidic urine + Acidic Drug drug remains non ionised Drug is reabsorbed • Weak basic drugs (morphine) excreted in acidic urine as highly ionised
- 36. Biliary Excretion and Entero Hepatic Circulation Liver transfers drugs to Bile intestine get reabsorbed, deconjugated or hydrolyses by gut enzymes releases parent active drug form. E.g. Digoxin, thyroxine, morphine , chloramphenicol, tetracycline, Ethinyl estradiol. Net effect – Prolongation of Drug Action
- 37. Faecal elimination Drugs which are not absorbed through gut eg. Streptomycin, mgso4 Drugs which are excreted through bile and not reabsorbed. Eg. Erythromycin, corticosteroids Alveolar excretion Gas and volatile liquids through breath depending upon their partial pressure and not lipid solubility.
- 38. Kinetics of drug elimination Half life PLASMA HALF LIFE (t1/2) • Time duration in which concentration of the drug falls by 50% than earlier value. • Reflects towards clearance kinetics • Helps to determine the DOSE SCHEDULE Biological half life • Time in which the principal action of the drug declines by half.
- 39. Aspects of drug elimination Following are the 3 important aspects of drug elimination • First-order kinetics:- for most drugs in their therapeutic concentration ranges, the amount of drug metabolized per unit time is proportional to the plasma concentration of the drug (cp ) and the fraction of drug removed by metabolism is constant (i.e., First-order kinetics).
- 40. Constant fraction (%) of drug eliminated over a constant interval of time. Rate of elimination ∞ Plasma Concentration T ½ remains constant irrespective of the dose Plasma Fall Out Curve • On Arithematic Scale - Curvilinear • On Log Scale – Linear
- 42. • After single dose 97 % of the drug gets eliminated after 5th t ½ • If fixed dose given 97 % of the drug gets eliminated on 5th t ½ after which the plasma concentration reaches a STEADY STATE CONCENTRATION Rate of absorption = rate of elimination Till then the plasma concentration rises. • If the dose is doubled, its duration of action is prolonged for 1 more half life.
- 43. • LOG PLASMA FALL OUT CURVE of drugs having high apparent Vd but obeying 1st order kinetics exhibit two slopes Distribution Half Life Elimination Half life • Derived from β- slope • Can be calculated t ½ = 0.693 K K – elimination rate constant Slope = -- K 2.303
- 44. • Zero-order kinetics for some drugs, such as ethanol and phenytoin, metabolic capacity is saturated at the concentrations usually employed, and drug metabolism becomes zero order; that is, a constant amount of drug is metabolized per unit time. Zero-order kinetics can also occur at high (toxic) concentrations as drug- metabolizing capacity becomes saturated.
- 45. • Same quantity/ Fixed quantity is eliminated per unit time. • Rate of elimination is not proportional to Plasma concentration. • T ½ is NEVER constant and is variable • Fall in Plasma Concentration against time (falling at constant rate) • Arithmatic scale – Steeply Linear • Log scale – Curvilinear
- 47. Michaelis Menten Kinetics/ Mixed Order/ Saturation Kinetics • Some drugs have a tendency to exhibit both the order of kinetics for elimination. • Dose dependent kinetics – • Smaller doses first order kinetics • When the plasma concentration (d/t dose) rises the drug follows zero order kinetics • A point comes where the enzymes get saturated resulting in the above.
- 48. The shift of the kinetics is risky and produces changes in t ½ on changing the dose. Proper monitoring and maintaining the plasma concentration is required or else causes Toxicity.
- 50. • The Aim of Drug treatment is to achieve Response without Adverse Effects on the Basis of Amount of drug administered and its relation with plasma Concentration • For drugs having longer t1/2 Like digoxin, diazepam, chloroquine, five half life are needed to reach steady state plasma concn.so in emergency like in CHF with atrial fibrillation ,delay is fatal. So an initial loading dose is given to achieve steady state plasma concn., then followed by maintenance dose to maintain Cpss.
- 51. • Inducible bio transforming enzymes:- the major drug-metabolizing systems are inducible, broad-spectrum enzymes with some predictable genetic variations. Drugs that are substrates in common for a metabolizing enzyme may interfere with each other’s metabolism, or a drug may induce or enhance metabolism of either of them.
- 54. Loading dose Single or few quickly repeated doses given in the beginning to attain target concentration rapidly. Thus, loading dose is governed only by V and not by CL or t½.
- 55. Maintenance dose The amount of drug given to maintain the steady state plasma concentration (Cpss) of drug at regular interval so as to maintain the elimination. So it depends on CL or half life