Ppt 1 overview of regulatory affairs and diff bodies_august2016_final
- 1. Overview of Regulatory Affairs and Worldwide Regulatory bodies Rajashri Survase-Ojha (Founder & Director, Raaj GPRAC) www.raajpharmaelearning.com rajashrio@gmail.com raajgprac@gmail.com
- 2. Disclaimer Contents of this presentation are the presenters personal views and do not necessarily represent any companies policies and position. Some images are taken – freely available from the internet for a diagrammatic representation of the content and the source is acknowledged. 2
- 3. What is RA? Regulatory Affairs (RA) is a profession within the health care industry namely, Pharmaceutical, Medical Device, Biologics, & Functional Food. Regulatory Affairs is a profession which has developed from the desire of governments to protect public health, by controlling the safety and efficacy of products in areas including pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines. RA profession at its heart is all about Collecting, Analyzing and Communicating the Risks and Benefits of health care products to regulatory agencies and public all over the world. RA can be defined as : It means government affairs 3
- 4. Role of RA Professionals RA as profession is broader than registration of products, they advise companies both strategically and technically at the highest level. Their role begins right from development of a product to making, marketing and post marketing. They advice at all stages both in terms of legal and technical requirements and restrains help companies save a lot of time and money in developing the product and marketing the same. They have a major contribution in company’s success both commercially and scientifically. Their main role is to comply with Safety & Efficacy of the products as per regulation laid down by the government. 4
- 5. Role of RA Professionals cont.. In an organization their prime responsibilities involves preparation and presentation of registration documents to regulatory agencies and carry out all following discussion to obtain and maintain marketing authorization (MA) for the products concerned. They need to keep a track on ever changing legislation in all countries where the companies is looking to market their product. They are responsible for the presentation of registration documents to regulatory agencies in India and importing countries and carry out all the subsequent negotiations necessary to obtain and maintain marketing authorization for the products concerned in country of origin as well as importing countries 5 5
- 6. Why RA?? The Pharmaceutical sector has been ever growing and with globalization the race to lead to be first is no more restricted by boundaries, companies need to dominate on a global level to stay on top. As a result of this competitiveness companies success lies in the “time taken” by the product to reach the market. The companies responsible for the discovery, testing, manufacture and marketing of these products also want to ensure that they supply products that are safe and make a worthwhile contribution to public health and welfare. Most companies, whether they are major multinational pharmaceutical corporations or small, innovative biotechnology companies, have special departments of Regulatory Affairs professionals 6
- 7. Why RA?? On an average it takes 15-20 years for a new drug development, and it cost around $800-1000 million. With such an expensive and time consuming activity Companies cannot afford a single day delay in getting the product to the market. RA Professional play the very important role in getting the product to market, improper data reporting can delay evaluation of a positive marketing authorization. 7
- 11. FDA is the federal agency responsible for ensuring that foods are safe, wholesome and sanitary; human and veterinary drugs, biological products, and medical devices are safe and effective; cosmetics are safe; and electronic products that emit radiation are safe. FDA also ensures that these products are honestly, accurately and informatively represented to the public. • Biologics Cosmetics Drugs Foods Medical devices Radiation Emitting Electronic products Veterinary Products What is FDA? What does FDA regulate?
- 12. • Advertising The Federal Trade Commission is the federal agency which regulates all advertising, excluding prescription drugs and medical devices. FTC ensures that advertisements are truthful and not misleading for consumers. • Alcohol The labeling and quality of alcoholic beverages are regulated by the Treasury Department's Bureau of Alcohol, Tobacco, and Firearms (ATFs). • Consumer Products While FDA regulates a large portion of the products that consumers purchase, the agency has no jurisdiction over many household goods. The Consumer Product Safety Commission (CPSC) is responsible for ensuring the safety of consumer goods such as household appliances (excluding those that emit radiation), paint, child-resistant packages, and baby toys. • Drugs of Abuse Illegal drugs with no approved medical use--such as heroin and marijuana--are under the jurisdiction of the Drug Enforcement Administration. • Health Insurance • FDA does not regulate health insurance, the cost of health care products or procedures, or reimbursement for health and medical expenses. Questions about Medicare should be directed to the Centers for Medicare and Medicaid Services. What FDA does not regulate?
- 13. What FDA does not regulate? Contd… Meat and Poultry The U.S. Department of Agriculture's Food Safety and Inspection Service is responsible for the safety and labeling of traditional meats and poultry. (FDA regulates game meats, such as venison, ostrich and snake.) • Pesticides FDA, USDA, and the Environmental Protection Agency share the responsibility for regulating pesticides. EPA determines the safety and effectiveness of the chemicals and establishes tolerance levels for residues on feed crops, as well as for raw and processed foods. • Restaurants and Grocery Stores Inspections and licensing of restaurants and grocery stores are typically handled by local county health departments. • Water The regulation of water is divided between the Environmental Protection Agency and FDA. EPA has the responsibility for developing national standards for drinking water from municipal water supplies. FDA regulates the labeling and safety of bottled water.
- 14. The US Regulatory Environment (1) The US Food and Drug Administration (FDA) FDA’s Role and Responsibilities: – FDA is regulating drugs, foods, cosmetics, biologics, medical devices – FDA is responsible for administration, enforcement, interpretation of US drug law and has power to establish regulations which have the force and effect of law – FDA has developed policies, procedures and regulations to implement its Reglatory initiatives, some going beyond the intent of the original laws Drug Registration in the US = one of the most rigorous systems in the world
- 15. Overview of FDA Organization – top level Office of the Commissioner (OC) Office of Regulatory Affairs (ORA) Centers for Product Jurisdiction CDER (Center for Drug Evaluation and Research) CBER (Center for Biologics Evaluation and Research) CDRH (Center for Devices and Radiological Health) CVM (Center for Veterinary Medicine) CFSAN (Center for Food Safety and Applied Nutrition) NCTR (National Center for Toxicological Research) The US Regulatory Environment (2)
- 16. 16'©"Raaj GPRAC, Mumbai 2010-2016. All rights reserved.''
- 19. Evolution of the European Regulatory Environment since 1995… 1995 2000 2003 2004 2005 2006 2007 EMEA Centralised Procedure Mutual Recognitio n Procedure Orphan Drugs Regulation Annex I (to Directive 2001/83/EC) (CTD) New Legisl. Title IV of Reg. 726/04 immediate New Legisl. fully into force Paediatric Regulatio n Legisl. on Advance d Therapy Enlargeme nt (to 15 MS) Enlargemen t to 25 MS (CY, CZ, EE, HU, LT, LV, MT, PL, SI, SK) Enlargemen t to 27 MS (BG & RO) Birth of theBirth of the EMEAEMEA 2008 Enlarged Scope of CP 2010 New Variation Regulation New Pharmacov igilance legislation Future… Accession of Croatia 2012 Implem. of New PV Legisl. PRAC (July 2012)
- 20. Austria (1995) Belgium (1952) Bulgaria (2007) Cyprus (2004) Czech Republic (2004) Denmark (1973) Estonia (2004) Finland (1995) France (1952) Germany (1952) Greece (1981) Hungary (2004) Ireland (1973) Croatia (2013) Italy (1952) Latvia (2004) Lithuania (2004) Luxembourg (1952) Malta (2004) Netherlands (1952) Poland (2004) Portugal (1986) Romania (2007) Slovakia (2004) Slovenia (2004) Spain (1986) Sweden (1995) United Kingdom (1973) 20 Member states of the European Union 08/25/16
- 21. COMP Committee on Orphan Medicinal Products COMP Committee on Orphan Medicinal Products CHMP Committee for Medicinal Products for Human Use CHMP Committee for Medicinal Products for Human Use HMPC Committee on Herbal Medicinal Products HMPC Committee on Herbal Medicinal Products NATIONAL COMPETENT AUTHORITIES ( > 3 500 EUROPEAN EXPERTS ) Structure of the European Medicines Agency (EMA) PRAC Pharmacovig. and Risk Assessment Committee PRAC Pharmacovig. and Risk Assessment Committee CVMP Committee for Medicinal Products for veterinary use CVMP Committee for Medicinal Products for veterinary use xpertsappointedbyNCA EXECUTIVE DIRECTOR Guido Rasi COMMUNICATIONS AND NETWORKING ADMINISTRATIONVETERINARY MEDICINES AND INSPECTIONS VETERINARY MEDICINES AND INSPECTIONS PATIENT HEALTH PROTECTION PATIENT HEALTH PROTECTION HUMAN MEDICINES DEVELOPMENT AND EVALUATION HUMAN MEDICINES DEVELOPMENT AND EVALUATION EMAPermanentStaff PDCO Paediatric Committee PDCO Paediatric Committee CAT Committee for Advanced Therapies CAT Committee for Advanced Therapies
- 22. • 27 MSs + NO/IS (EEA Countries) - 22 Languages (Working Language EN) • 1 scientific expert member nominated by each MS and 1 alternate • 1 scientific expert member from NO and IS and 1 alternate (observers) • 5 co-opted members as appointed by Management Board • 3 years Mandate renewable Composition Chairman (Dr. T. Salmonson; SE) & Vice-Chairman (Dr. I. Hudson; UK) CHMP (Committee for Human Medicinal Products)
- 23. Delivering opinions to the European Commission on new medicinal products/variation/renewal/line extension on arbitration/referral procedures • Advising on general EU guidelines/policies • Scientific Advice & guidelines to companies • Opinions on Compassionate Use to MSs • Contribution to ICH process • Establish Working Parties, SAGs & Expert Groups • Delivering opinions to WHO CHMP main tasks & responsibilities
- 24. Working Party Constellation CHMP QWP* SWP* BWP*Sci Adv PhVig* Patients & Consumers Biosimilars tWP Biostatistics tWP Blood Prod. tWP Cardiovascular tWP CNS tWP Rheumatology Immunology tWP Infectious Diseases tWP Oncology tWP Pharmacokinetics tWP Pharmacogenomic s tWP Vaccines tWP Gastroenterology DG Respiratory DG Urology DG Radiopharmaceuticals DG QSE standards Evaluation of Medicines * 1 / MS representation SAG diagnostics SAG CVS SAG Neurology SAG Psychiatry SAG Diabetes SAG HIV / Antiviral SAG Anti-Infectives SAG Oncology DG = drafting groups tWP = temporary working party SAG Vaccines
- 25. CompositionComposition Chair and Vice Chair + 1 member/MS + 3 reps patient organizations + 3 EMA reps + 3 patient organisations + 3 EMA COMP (Committee for Orphan Medicinal Products) Dr. Westermark
- 26. 26 Composition: Chair and Vice Chair + 5 CHMP members + 1 member/MS (not yet represented by member appointed by CHMP) + 3 reps patient organizations + 3 reps healthcare professionals + 3 patient organisations + 3 healthcare prof. Dr. D. Brasseur PDCO (Paediatric Committee)
- 27. 27 Registration in Europe Post Nov 2005 : Three European Systems Centralised Procedure (via EMA) Mutual Recognition procedure Decentralised Procedure
- 28. EU Centralised Procedure Legal Basis: Regul. (EC) No 726/2004 (also establishing “EMA” European Medicines Agency) Principle: single application / evaluation single authorisation direct access to all EU(27MSs) + Norway, Iceland and Liechtenstein Scope: Compulsory for: Biotech (recombinant DNA, gene expressed proteins, hybridoma & monoclonal antibodies) New Active Substances in Specific Therapy Areas: AIDS, Cancer, Neuro-degenerative disorder, Diabetes, Auto-immune disease, other immune deficiencies, Viral diseases Orphan Drugs Optional for: Any Other New Active Substance significant innovation (therapeutic, scientific or technical) in the interests of patients at community level Generic of Centralised reference prod. (may use CP or MRP/DCP)
- 29. Project management and co-ordination Industry •Rapporteur/Co-rapporteur •Scientific evaluation - experts •Opinion and assessment report •Working parties EMA CHMP EU Commissio nDriving & adoption of the decision (EU license) Members states1 per MS + 5 Co-opted members. Each MS has an Alternative. Applicant MAH
- 30. Procedures for evaluating medicinal products and granting marketing authorization The European system for the authorisation of medicinal products for human and animal use was introduced in January 1995 with the objective of ensuring that safe, effective and high quality medicines could quickly be made available to citizens across the European Union. The European system offers several routes for the authorisation of medicinal products: 30 '©"RaajGPRAC,Mumbai2010-2016.Allrightsreserved.''
- 31. Centralised procedure The centralised procedure, which is compulsory for products derived from biotechnology, for orphan medicinal products and for medicinal products for human use which contain an active substance authorised in the Community after 20 May 2004 (date of entry into force of Regulation (EC) No 726/2004) and which are intended for the treatment of AIDS, cancer, neurodegenerative disorders or diabetes. The centralised procedure is also mandatory for veterinary medicinal products intended primarily for use as performance enhancers in order to promote growth or to increase yields from treated animals. Applications for the centralised procedure are made directly to the European Medicines Agency (EMA) and lead to the granting of a European marketing authorisation by the Commission which is binding in all Member States. 31 '©"RaajGPRAC,Mumbai2010-2016.Allrightsreserved.''
- 32. MRP & DCP The mutual recognition procedure, which is applicable to the majority of conventional medicinal products, is based on the principle of recognition of an already existing national marketing authorisation by one or more Member States. The decentralised procedure, which was introduced with the legislative review of 2004, is also applicable to the majority of conventional medicinal products. Through this procedure an application for the marketing authorisation of a medicinal product is submitted simultaneously in several Member States, one of them being chosen as the "Reference Member State". At the end of the procedure national marketing authorisations are granted in the reference and in the concerned Member States. 32 '©"RaajGPRAC,Mumbai2010-2016.Allrightsreserved.''
- 33. The 3 steps of the Centralised Procedure Step I Pre-submission to application • Early advice • Rapporteur/C-rapporteur appoinmt • Assessment team • Application • Validation Step II Scientific evaluation • Assessment Reports • List of Questions (+ clock stop) • CHMP Opinion • Possibility to appeal • Transfer to EU Commission Step III Decision Making Process -120 days 210 days 67 days Notify the EMA Request Rapporteur/co-Rapporteur Submit the MAA CHMP Opinion Draft EC Decision EC Decision Community Licence favourable Answer to questions Oral hearing Clock stop for answer to questions
- 34. Two options Mutual Recognition Procedure Art. 28 para. 2 of Dir. 2001/83/EC For products with an existing MA Decentralised Procedure Art. 28 para. 3 of Dir. 2001/83/EC Only possible, if no authorisation has already been granted Most significant difference with MRP = consultation between MS‘s before 1st MA issued &:
- 35. MRP & DCP: key authority stakeholders CMDh ("Coordination group for mutual recognition and decentralised procedure for human medicinal products"): Mixed responsibilities: procedural, regulatory and scientific One representative from each MS, appointed for 3 years (renewable) + observer from EMA and Commission CMD(h) Chair appointed for 3 years + Vice-chair representative of MS that has presidency of Council RMS ("Reference Member State") Selected by the applicant Has to prepare the assessment report (AR) Acts as central point between MS and MAH CMS ("Concerned Member State(s)") All other MS‘s where the Company has submitted the dossier
- 36. Overview of the MRP Application to Reference Member State (RMS) RMS Approval (Day 210) Submit MR application to Member States National Submission 210 days Mutual Recognition 90 days Closure of procedure (AR, SPC, labelling, PIL) Arbitration by CHMP (Art.32, 33, 34) 60 days (CHMP opinion) + 30 days (Commission decision) National licences granted within MSs Approval Arbitration By CMDh (60 days) CMDh National Step (30 days) Serious objections Referral to CMDh ApprovalSerious objections Referral to CHMP
- 37. Overview of the DCP Submission of dossier to Reference Member State (RMS) and Concerned Member States (CMSs) RMS starts evaluation, and issues preliminary Assessment Report (AR) for comments by CMSs RMS sends consolidated list of questions to Applicant RMS prepares draft AR, draft SPC and draft labelling/package leaflet Submit MR application to Member States Closure of procedure (AR, SPC, labelling, PIL) Arbitration by CHMP (Art.32, 33, 34) 60 days (CHMP opinion) + 30 days (Commission decision) National licences granted within MSs Approval DCP Step1 120 days DCP Step 2 90 days Arbitration By CMDh (60 days) CMDh Clock stop National Step (30 days) Serious objections Referral to CMDh ApprovalSerious objections Referral to CHMP (recommended 3 mths)
- 38. The “Pharmacovigilance and Risk Assessment Committee” (PRAC) Replacing Pharmacovigilance Working Party (PhV WP) Mandate: Risk detection, Benefit-Risk Assessment, Communication of risk and benefit/risk, Risk Minimisation and Analysis Impact, Design and Evaluation of PASS CHMP & CMDh to “rely upon” recommendations from PRAC but retain responsibility for benefit-risk assessments PRAC started in July 2012 Membership: 1 member (& 1 alternate) from each MS Chair: June M. Raine (UK – MHRA) Vice-Chair: Almath Spooner (Ireland – IMB) Interaction between PRAC & CHMP: About 30% of CHMP agenda would go to PRAC Aiming that PRAC Rapporteur could come from same MS as CHMP Rapporteur Chalenge with timing of PRAC opinions: i.e. trying to fit the PRAC 60 day review timeframe into overall timelines and still allow CHMP time to consider PRAC input before adopting their opinion
- 39. New requirements for the Marketing Authorisation Application (MAA) Pharmacovigilance System Master File (PSMF) MAH must prepare and maintain “Pharmacovigilance system master file” (PSMF): replaces former “Detailed Description of Pharmacovigilance System” (DDPS), and must be held at MAH site Summary of the Pharmacovigilance System must be included in the MAA dossier Full PSMF to be provided within 7 days of request from a competent authority Risk Management Plan (RMP) ‘detailed description of risk management system’ RMPs to be included in MAAs for all new products N.B. Authorities can impose need for Risk Management System on already authorised products if concerns about Benefit/Risk balance Format and content of RMPs addressed in Commission’s implementing measures
- 40. Overview of the Regulation-EU EU Regulatory system Assessment on MAA (Agencies-EMEA) [www.eudra.org/emea] EDQM/Ph.Eur (Council of Europe) [www.pheur.org] Volume 1: Pharmaceutical Legislation for Medicinal Products for Human use Volume 2: Notice to Applicants for MP for Human use 2A Procedures for marketing Authorization 2B Presentation and format of the dossier(CTD) Volume 3: Guidelines 3A Quality and Biotechnology 3B Safety, environment and information 3C Efficacy Volume 4: Good Manufacturing Practices Volume 5: Pharmaceutical Legislation for Medicinal Products for Veterinary use Volume 6: Notice to Applicants for MP for Veterinary use Volume 7: Scientific guidelines for MP for Veterinary use Volume 8: Maximum residue limits Volume 9: Guidelines for PV for MP for Human and Veterinary use Volume 10: Guidelines for Clinical Trial Laws, Directives, regulations (EU institutions) [http://pharmacos.eudra.org] Relevant EU legislation/ Guidelines
- 41. Maintenance of Marketing Authorizations (Variations, Renewals) EU Variations Commission Guideline on Dossier Requirements for Type IA & IB Notifications, July 2006 Categories of Variations Minor Changes Type IAIN variation and Type IA Type IB variation (Notify, wait for 30 days ) Type II variation (Needs prior approval, 60 days evaluation time period) Major Changes Differences between the categories • Approval time • Documentation to be submitted • Money(Fees)
- 42. The Medicines Control Council (MCC) is a statutory body that was established in terms of the Medicines and Related Substances Control Act, 101 of 1965, to oversee the regulation of medicines in South Africa. It is appointed by the Minister of Health and its main purpose is to safeguard and protect the public through ensuring that all medicines that are sold and used in South Africa are safe, therapeutically effective and consistently meet acceptable standards of quality. South Africa : MEDICINES CONTROL COUNCIL(MCC)
- 43. MCC Structure A F R IC A N T R A D I T IO N A L M E D I C I N E S C O M M IT T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n C O M P L E M E N T A R Y M E D I C I N E S C O M M I T T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n V E T E R I N A R Y C L I N I C A L C O M M I T T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n A N A L Y T I C A L C O M M I T T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n B I O L O G IC A L C O M M IT T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n C L I N I C A L C O M M I T T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n C L I N I C A L T R I A L S C O M M I T T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n P H A R M A C E U T I C A L / B I O A V A I L A B IL I T Y C O M M I T T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n P H A R M A C O V I G I L A N C E C O M M I T T E C h a i r p e r s o n V i c e - C h a i r p e r s o n S C H E D U L IN G C O M M I T T E E C h a i r p e r s o n V i c e - C h a i r p e r s o n M E D I C I N E S C O N T R O L C O U N C IL C h a i r p e r s o n V i c e - C h a i r p e r s o n South Africa : MEDICINES CONTROL COUNCIL(MCC)
- 44. UK-MHRA: Medicines and Healthcare products Regulatory Agency The MHRA regulates a wide range of materials like; Medicines Devices Tissue Engineering Nanotechnology Blood The term “medicines” embraces both pharmaceutical and biological medicines, and vaccines. The term “medical devices” includes medical equipment. Medical devices are all products, except medicines, used in healthcare for the diagnosis, prevention, monitoring or treatment of illness or disability. Examples include X-ray and other imaging equipment, pacemakers, artificial joints, anaesthetic equipment, pregnancy test kits, infusion equipment, beds, wheelchairs, condoms and surgical dressings.
- 45. WHO: World Health Organization WHO is the directing and coordinating authority for health within the United Nations system. It is responsible for providing leadership on global health matters, shaping the health research agenda, setting norms and standards, articulating evidence-based policy options, providing technical support to countries and monitoring and assessing health trends. WHO is Geneva based. Many countries follow the guidelines provided by WHO. Food and Drugs Control Authority India follows WHO guidelines. WHO GMP certification is a requirement and well accepted in most of the countries across Asia, Africa, CIS and Latin America.
- 46. How does WHO work and for whom? WHO’s main functions: - To act as the directing and coordinating authority on international health work - To encourage technical cooperation for health with Member States - Has constitutional mandate to develop norms and standards
- 47. Countries All countries which are Members of the United Nations may become members of WHO by accepting its Constitution. Other countries may be admitted as members when their application has been approved by a simple majority vote of the World Health Assembly. Territories which are not responsible for the conduct of their international relations may be admitted as Associate Members upon application made on their behalf by the Member or other authority responsible for their international relations. Members of WHO are grouped according to regional distribution (193 Member States). WHO: World Health Organization
- 49. Japanese law and relevant authorities for medicines registration Pharmaceutical Affairs Law (PAL) – April 2005 MHLW (Ministry of Health, Labour and Welfare) – Responsibility for approvals and licensing – http://www.mhlw.go.jp/english/index.html PMDA (Pharmaceuticals and Medical Devices Agency) – Responsibility for scientific review (incl. audit for GMP, GLP, GCP) – Branch under MHLW, created in 2004 – http://www.pmda.go.jp/english/index.html NIID (National Institute of Infectious Diseases) – Responsibility for national testing – http://www.nih.go.jp/niid/index-e.html
- 50. Relationship between MHLW and PMDA Applicant MHLW Advisory Committee Consultation AdviceApproval Pharmaceuticals and Medical Devices Agency (PMDA) Consultation and Review (Single Review Team) External Experts Compliance Review etc Questions and Responses Consultation and NDA Review Report Instruction Expert Discussion
- 52. Scope 192 countries 23 time zones Between 4000 and 6000 languages More than 150 monetary currencies More than 70% of the world’s population are: Asia Pacific and Emerging Markets (APEM )
- 53. International & Emerging Regions Asia Pacific: India, Philippines, South Korea, Taiwan China / Hong Kong / Macau: China Latin America : Argentina, Brazil, Chile, Mexico Middle East and North Africa (MENA): Egypt, Pakistan, Saudi Arabia, Turkey Sub Saharan Africa (SSA): South Africa Eastern Europe: Key markets
- 54. Models for drug regulatory assessment Countries can be categorised in 2 licensing models: model of licensing system based upon submission of evidence of registration in reference countries model of licensing system based upon a full assessment of new drug applications (including biological products)
- 55. RoW = Rest of the World What are the RoW countries? World regions excluding US, CA, EU, CH, Au, and Nz. What are the regions and sub-regions? AMEA – Asia Middle East and English-speaking Africa LATAM – Latin America RIC+ - Russia & Former Soviet States, Greater China, Greater India, Israel, French-speaking Africa, South-east Europe
- 56. Regions and Sub-regions - definitions AMEA ASIA ASEAN (Association of South East Asian Nations) Korea, Australasia (AUS, NZ), Japan MIDDLE EAST Arabian Peninsula (Saudi Arabia) Gulf (Bahrain, Kuwait, Qatar, UAE, Oman, Yemen) Near East (Egypt, Jordan, Lebanon, Syria, Irak, Iran, Afghanistan, Pakistan) AFRICA English-speaking Africa
- 57. Region ASEA – definition ASEAN (Association of South East Asian Nations) Singapore, Malaysia, Thailand, Philippines, Indonesia, Laos, Cambodia, Vietnam , Brunei Darussalam, Myanmar
- 58. ASEAN Association of Southeast Asian Nations 10 member states : Indonesia, Malaysia, Philippines, Singapore, Thailand, Brunei, Vietnam, Laos, Myanmar, Cambodia Population of about 560 million, a total area of 4.5 million square kilometers, a combined GDP of US$ 1,100 billion, and a total trade of about US$ 1,400 billion Objectives : To accelerate economic growth, social progress and cultural development in the region and to promote regional peace and stability
- 59. ASEAN: 2 main areas of Harmonisation ACTD (ASEAN Common Technical Dossier) : > Harmonize format ACTR (ASEAN Common Technical Requirement) :> Harmonize technical content Developed based on international guidelines such as ICH, EMEA, FDA, WHO
- 60. ASEAN: Organization of Application Dossier (ACTD) * Upon RequestPart I Administrative Data & Product Information Part II Quality Overall Summary & Reports Part III Non-clinical Overview, Summary & Study Reports* Part IV Clinical Overview, Summary & Study Reports*
- 61. Region AMEA – definition ENGLISH-SPEAKING AFRICA Angola, Ethiopia, Ghana, Kenya, Malawi, Mozambique, Namibia, Nigeria, Sierra Leone, Somalia, South Africa,Tanzania, Uganda, Zambia, Zimbabwe
- 62. Region RIC+ RIC+ RUSSIA AND FORMER SOVIET UNION COUNTRIES Russia Ukraine OFSUs (Armenia, Azerbaijan, Belarus, Georgia, Kazakhstan, Kirghistan, Moldova, Tajikistan, Turkmenistan, Uzbekistan) GREATER INDIA (India, Sri Lanka, Bangladesh) GREATER CHINA (China, Hong Kong, Taiwan) ISRAEL FRENCH-SPEAKING AFRICA SOUTH-EAST EUROPE (Albania, Bosnia-Herzegovina, Kosovo, Macedonia, Montenegro, Serbia, Srpska)
- 63. Region RIC+ 1. Armenia 2. Azerbaijan 3. Belarus 5. Georgia 6. Kazakhstan 7. Kirghistan 10. Moldova 11. Russia 12. Tajikistan 13. Turkmenistan 14. Ukraine 15. Uzbekistan RUSSIA AND FSU COUNTRIES
- 64. Region RIC+ FRENCH-SPEAKING AFRICA Algeria, Benin, Burkina Faso, Cameroon, Chad, Congo Brazzaville, Congo Kinshasa, Ethiopia, Gabon, Guinea, Ivory Coast, Madagascar, Mali, Mauretania, Mauritius, Morocco, Rep. Central Africa, Senegal, Togo, Tunisia (Arabic-speaking Africa : Libya, Sudan )
- 65. General Overview of specific regional requirements Regions, but all are national registrations Enormous diversity of regulatory requirements However, some harmonisation on some clusters e.g. ASEAN, Gulf Currently, the registration documentation can be either EU-based files, complemented with additional HA’s requirements, OR CTD files specifically prepared for geographical expansion in AMEA, LATAM, RIC+ CTD format is not accepted in all RoW countries but dossier must be submitted as per respective HA guidelines.
- 66. General Overview of specific regional requirements When a standard file is sent to RoW countries for submission, Regulatory dept. often receives a list of additional documents needed before the file can be submitted to the HA. Collecting these documents can take weeks/months and further delay the submission. Many countries insist on additional specific documentation for product registration, variations, site changes, etc. Mostly, the additional requirements are of a CMC nature, although there are requests for extra certificates, statements, CPPs, etc
- 67. GCC-Middle East Countries Armenia Israel Syria Azerbaijan Jordan Turkey Bahrain Kuwait Turkmenistan Egypt Lebanon United Arab Emirates Georgia Oman Yemen Iran Qatar Iraq Saudi Arabia
- 68. Middle East countries developed legislations for drug registrations, inspection, drug control and life cycle. Legislation and review process varies from country to country. Classification of the products varies to the degree of development (new chemical entities, generic, similar etc.) Background:
- 70. Local (Domestic market) Registration of products in our own country India for selling to Local market Regulatory Authority :Food and Drug Administration (FDA) Reference : Drugs and cosmetic Act 1940, Rules 1945 70
- 71. Regulatory Bodies Governing & Controlling Clinical Trials Regulatory Body Ministry Location Drugs Controller General (India) (DCGI), Directorate General of Health Services (DGHS) Ministry of Health and Family Welfare Central Body (Delhi)
- 72. Local (Domestic market) New Molecule Registration of new products in our own country -India for selling to Local market Regulatory Authority :Drug Control General (DCGI),Delhi Reference : Drugs and cosmetic Act 1940. Rules 1945. 72
- 74. What is ICH? “International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use”. ICH is a joint initiative involving both regulators and research-based industry representatives of the EU, Japan and the US in scientific and technical discussions of the testing procedures required to assess and ensure the safety, quality and efficacy of medicines.
- 75. ICH Structure / Organisation 6 official parties (co-sponsors) directly involved : Authorities and research based industry from EU:- European Commission + EMA and CHMP - EFPIA (Eur.Feder.Pharmac.Industries&Associations) Japan:- MHLW (Ministry of Health and Welfare) - JPMA (Japanese Pharmaceutical Manuf. Association) USA:- FDA (US Food and Drug Administration) - PhRMA (Pharmaceut. Research and Manuf. of America) Official "Observers" World Health Organisation (WHO) European Free Trade Area (EFTA) Canada (Health Protection Branch) "Interested Parties" also involved Pharmacopoeias (Eur.Ph., J.P., U.S.P.) other industry sectors (OTC and Generics)
- 76. What is the purpose of ICH? The objective of ICH is to increase international harmonization of technical requirements to ensure that safe, effective, and high quality medicines are developed and registered in the most efficient and cost effective manner.
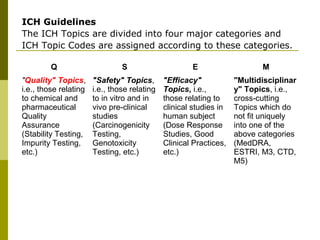
- 77. ICH Guidelines The ICH Topics are divided into four major categories and ICH Topic Codes are assigned according to these categories. Q S E M "Quality" Topics, i.e., those relating to chemical and pharmaceutical Quality Assurance (Stability Testing, Impurity Testing, etc.) "Safety" Topics, i.e., those relating to in vitro and in vivo pre-clinical studies (Carcinogenicity Testing, Genotoxicity Testing, etc.) "Efficacy" Topics, i.e., those relating to clinical studies in human subject (Dose Response Studies, Good Clinical Practices, etc.) "Multidisciplinar y" Topics, i.e., cross-cutting Topics which do not fit uniquely into one of the above categories (MedDRA, ESTRI, M3, CTD, M5)
- 78. Stability - Q1A – Q1F Analytical Validation – Q2 Impurities – Q3A - Q3C (Q3D – concept paper) Pharmacopoeias – Q4A - Q4B (and annexes) Quality of Biotechnological Products – Q5A – Q5E Specifications – Q6A – Q6B Good Manufacturing Practice – Q7 Pharmaceutical Development – Q8 Quality Risk Management - Q9 Pharmaceutical Quality System – Q10 Development and Manufacturing of Drug Substances – Q11 "Quality" Topics
- 79. Efficacy Topics E1: The Extent of Population Exposure to Assess Clinical Safety for Drugs Intended for Long- Term Treatment of Non-Life-Threatening Conditions E2A: Clinical Safety Data Management : Definitions and Standards for Expedited ReportingE3: Structure and Content of Clinical Study Reports E3: Structure and Content of Clinical Study Reports E4: Dose-Response Information to Support Drug Registration E5(R1): Ethnic Factors in the Acceptability of Foreign Clinical Data E6: Good Clinical Practice : Consolidated Guideline E7: Studies in Support of Special Populations : Geriatrics E8: General Considerations for Clinical Trials E9: Statistical Principles for Clinical Trials E10: Choice of Control Group and Related Issues in Clinical Trials E11: Clinical Investigation of Medicinal Products in the Pediatric Population E12: Principles for Clinical Evaluation of New Antihypertensive Drugs E14: The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs E15: Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories E16: Genomic Biomarkers Related to Drug Response: Context, Structure and Format of Qualification Submissions
- 80. Multidisciplinary Guidelines M1 MedDRA Medical Terminology M2 ESTRI Electronic Standards for the Transfer of Regulatory Information M3M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals M4CTD The Common Technical Document M5M5 Data Elements and Standards for Drug Dictionaries
- 81. ICH Now and in the Future New areas to develop ICH guidelines: Post-marketing pharmacovigilance Pharmacogenomics Biomarkers Gene Therapy Continued Harmonisation: prevention of new interregional disharmony Avoid unilateral development of requirements in specific areas
- 82. …Globalising ICH ICH Global Cooperation Group (GCG) Representatives from other Regional Harmonisation initiatives APEC (Asia-Pacific Economic Cooperation) ASEAN (Association of the Southeast Asian Nations) GCC (Gulf Cooperation Council) PANDRH (Pan American Network for Drug Regulatory Harmonization) SADC (Southern African Development Community) Evolution in ICH and understanding that some non-ICH countries are now major contributors/consumers to the global pharmaceutical market Regulators Forum - started in 2008 Including representatives from Australia, Brazil, China, Taiwan, India, Russia, Singapore and South Korea most of which are not part of Harmonisation Initiatives already Key areas of focus: API GMP; clinical trials; pharmacovigilance
- 83. USA : UNITED STATES FOOD AND DRUG ADMINISTRATION http://www.fda.gov/ Europe : THE EUROPEAN AGENCY FOR THE EVALUATION OF THE MEDICINAL PRODUCTS (EMEA) http://www.emea.europa.eu/ Health Canada: http://www.hc-sc.gc.ca/dhp-mps/index-eng.php South Africa : MEDICINES CONTROL COUNCIL (MCC) http://www.mccza.com/ United Kingdom : MEDICINES AND HEALTHCARE PRODUCTS REGULATORY AGENCY (MHRA) http://www.mhra.gov.uk/ Various links
- 84. New Zealand : NEW ZEALAND MEDICINES AND MEDICAL DEVICES SAFETY AUTHORITY (MEDSAFE) http://www.medsafe.govt.nz/ Australia : THERAPEUTIC GOODS ADMINISTRATION (TGA) http://www.tga.gov.au/ Brazil: ANVISA http://www.anvisa.gov.br/eng/legis/index.htm#6 Various links
- 85. Thanks for your Attention!
Editor's Notes
- #3: This is the famous disclaimer.
- #20: Why do we need a corporate identity now, after 13 years of operating without one? According to professional 'brand consultants', whenever an organisation has undergone a radical change in its operations, is targeting a new market, or has simply outgrown its former self, it's time to review its corporate identity. When the Agency began its operations in 1995, it was a small agency with fewer than 70 staff members, had two scientific committees, relatively few core activities, and engaged with just two primary stakeholders (industry and regulatory bodies). Thirteen years on, the Agency has seven times the number of staff members, is currently creating its sixth scientific committee, has a vastly wider scope of responsibilities stemming from EU legislation, and is actively engaging with additional primary stakeholders (patients' and healthcare professionals' representatives). The Agency's existing corporate identity has not kept up with this very rapid pace of evolution, and is no longer 'fit for purpose'. (Partly because it was never formally defined in the first place.) It is therefore time, now, to develop a fully functional corporate identity that accurately reflects the nature of the Agency's operating environment today, helps us to engage with our audiences in a more professional and meaningful way, and supports the long-term strategic objectives of the Agency.
- #26: The Committee for Orphan Medicinal Products recommends whether a product should be designated as an orphan as under Regulation EC 141/2000. NOT THE REVIEW This Committee is unusual in that it is the only one which has representatives from patient organisations. In fact one of the patient organisation representatives is the vice chair at present. The EMEA representatives include 2 CHMP members and one person from the European Parliament (Abadie/Lyons/Benzi) Chair is Josep Torrent I Farnell DK Heidrum Bosch-Traberg Again following the inclusion of the OD legislation in the EEA Agreement, NO/IC and LI can participate in the COMP since July 2003 EMEA Scientific Committee: 33 members and 1 Chairman 1 Member per Member State 3 representatives from patients groups 3 members proposed by the EMEA COMP Responsible for: opinions on orphan designation advising Commission on orphan EU policy international co-operation
- #27: The Committee for Orphan Medicinal Products recommends whether a product should be designated as an orphan as under Regulation EC 141/2000. NOT THE REVIEW This Committee is unusual in that it is the only one which has representatives from patient organisations. In fact one of the patient organisation representatives is the vice chair at present. The EMEA representatives include 2 CHMP members and one person from the European Parliament (Abadie/Lyons/Benzi) Chair is Josep Torrent I Farnell DK Heidrum Bosch-Traberg Again following the inclusion of the OD legislation in the EEA Agreement, NO/IC and LI can participate in the COMP since July 2003 27 Members (plus alternates) including 5 from Approval Committee (CHMP) 3 HealthCare Professionals 3 Patients representatives 2 from Norway, Iceland 1 Chair elected
- #30: Centralised procedure Key Points: ONE licence for the whole of the EU ( application co-ordinated by EMA, one trademark per licence, identical prescribing information and labelling in all countries ( exception is country specific info. Eg. price/legal status that can be placed in a blue box on the outer packaging). Procedure is currently only available for certain types of products: List A – biotech (mandatory) List B – innovative medicinal products, (eg AIDS), new NCEs, or new indications of significant therapeutic interest. It is possible for the CPMP/Commission to force a company to use the centralised procedure, recently happened with lamividine. Procedure is co-ordinated by EMA. Also a time-table, but with clock stops. Overall approval time is longer than MR, typically around 15 months. Applicant proposes co-ordinating agencies but CPMP make final choice – known as rapporteur and co-rapporteur. These assess application and produce assessment report which is circulated to all other member states. These just receive summary of the dossier ( Part I) from the applicant. They raise Q’s and rapporteur issues list to applicant. Applicant responds, CPMP issues opinion. Maybe further clock stop for oral hearing if the application is problematic. Approval endorsed by the Commission, finally EMA issues licence. Time frame is 210 days for assessment (plus clock stop), followed by 110 days for commission endorsement/issue of licence by EMA.
- #34: After validation by the EMA the review of the application by the Rapporteurs begins. The EMA will ensure that the opinion of the CPMP is given within a maximum of 210 days. However within the 210 days several activities are undertaken. At Day 70 the Rapporteurs circulate a draft assessment report(s) to the other members of the CPMP. The amount of collusion between the rapporteurs depends on the particular authorities involved. The CPMP members then review the draft assessment report and circulate comments. During this process a draft list of questions is generated. During the review the Rapporteurs are allowed to laise with the applicant in order to clarify any specific issues relating to the data submitted. The amount of liason again can again depend on the particular auhtorities involved. By day 120 the CPMP validates the list of questions as well as the overall conclusions of the review and this is sent to the applicant. At this point the clock stops whilst the applicant prepares responses to the questions. Hence the applicant can influence review time. When the applicant submits their responses to the consolidated list of questions, this is day 121 and the clock starts again. By day 210 the responses will have been reviewed and discussed by the CPMP. On Day 210 the CPMP will give their opinion and and a draft assessment report. The opinion can be positive, conditional or negative. The opinion itself is not legally binding, only the EU Commission can turn this into a legally binding decision across the 15 EU member states and hence the opinion must then enter the Commission decision making process.
- #37: The process begins with a Marketing Authorisation application being submitted in the first member state, the reference member state. The application is reviewed by the member state authority within the permitted time of 210 days. If the reference member state grants approval, then an application for mutual recognition can be submitted to the other EU member states, those as identified by the applicant. During the mutual recognition phase the member states are requested to recognise the approval of the reference member state. If the other member states have any concerns with respect to the quality, safety and efficacy of the product these concerns are raised and discussed with both the reference member state and the applicant. 90 days are allowed for this dialogue and clarification phase of the mutual recognition process. If there are no serious objections remaining at the end of the 90 days, then national licences are granted within 30 days. If serious objections remain at the end of the 90 days, then the applicant can either withdraw the application from the particular member state or allow the application to go through the arbitration process.
- #38: The process begins with a Marketing Authorisation application being submitted in the first member state, the reference member state. The application is reviewed by the member state authority within the permitted time of 210 days. If the reference member state grants approval, then an application for mutual recognition can be submitted to the other EU member states, those as identified by the applicant. During the mutual recognition phase the member states are requested to recognise the approval of the reference member state. If the other member states have any concerns with respect to the quality, safety and efficacy of the product these concerns are raised and discussed with both the reference member state and the applicant. 90 days are allowed for this dialogue and clarification phase of the mutual recognition process. If there are no serious objections remaining at the end of the 90 days, then national licences are granted within 30 days. If serious objections remain at the end of the 90 days, then the applicant can either withdraw the application from the particular member state or allow the application to go through the arbitration process.

















![EU Regulatory
Environment [EMA]](https://image.slidesharecdn.com/ppt1overviewofregulatoryaffairsdiffbodiesaugust2016final-160825070643/85/Ppt-1-overview-of-regulatory-affairs-and-diff-bodies_august2016_final-17-320.jpg)






















![Overview of the Regulation-EU
EU Regulatory system
Assessment on MAA
(Agencies-EMEA)
[www.eudra.org/emea]
EDQM/Ph.Eur
(Council of Europe)
[www.pheur.org]
Volume 1: Pharmaceutical Legislation for Medicinal
Products for Human use
Volume 2: Notice to Applicants for MP for Human use
2A Procedures for marketing Authorization
2B Presentation and format of the dossier(CTD)
Volume 3: Guidelines
3A Quality and Biotechnology
3B Safety, environment and information
3C Efficacy
Volume 4: Good Manufacturing Practices
Volume 5: Pharmaceutical Legislation for Medicinal
Products for Veterinary use
Volume 6: Notice to Applicants for MP for Veterinary
use
Volume 7: Scientific guidelines for MP for Veterinary
use
Volume 8: Maximum residue limits
Volume 9: Guidelines for PV for MP for Human and
Veterinary use
Volume 10: Guidelines for Clinical Trial
Laws, Directives, regulations (EU institutions)
[http://pharmacos.eudra.org]
Relevant EU legislation/ Guidelines](https://image.slidesharecdn.com/ppt1overviewofregulatoryaffairsdiffbodiesaugust2016final-160825070643/85/Ppt-1-overview-of-regulatory-affairs-and-diff-bodies_august2016_final-40-320.jpg)