Session One: Risk Assessment
- 1. Genomics for the Child Neurologist: Genetic Risk Assessment
- 3. Neurologic conditions are complex diseases
- 4. Spectrum of Genetic Contribution GENEGENE Frequency Penetrance GENEGENE GENEGENE
- 5. Neurological conditions encompass all inheritance patterns Autosomal Dominant X-Linked
- 6. Workshop One: Genetics & Risk Assessment
- 7. Risk Assessment Learning Objectives 1. Take a family history with sufficient detail 2. Recognize genetic “red flags” 3. Analyze a detailed family history 4. Develop an appropriate evaluation plan based on personal and family history assessment 5. Communicate with families about genetic information
- 8. GENOMIC risk assessment expands on the family history Ask the right questions Identify red flags Identify patterns
- 9. Ask the right questions
- 10. 3 yo male Developmental delay FamHx: negative for ID/DD Clinical Scenario: Joseph
- 11. History Birth History: •normal Developmental History: •hearing normal •gross motor milestones on time •cannot dress himself •speech delay •behavioral problems, such as hyperactivity and temper tantrums Social History: •Joseph lives with his parents and his older sister •both English and Spanish are spoken in the home Past Medical History: •unremarkable
- 12. What questions would you ask about Joseph’s family history? Please check next to any condition that affects your child, parent, sibling, aunt/uncle or grandparent. For each checked box, explain below who is affected. Mental retardation Learning disabilities X Developmental delay Joseph has speech delay Early-onset deafness Early-onset blindness Birth defects Neuromuscular Issues Seizures X Abnormal movements Mom’s uncle has a tremor, ataxia, and maybe dementia Blood clotting or bleeding disorder Infant death Pregnancy losses Unexplained death Migraine X Cancer Paternal grandmother had breast cancer
- 14. • Family structure • Presence and extent of disease • Ages of onset and death • Chronic illness • Infertility, miscarriage • Intellectual disability • Birth defects Questions for a targeted famhx include:
- 15. Medical and development information about your relatives can give us clues about what is going on with Joseph. Even a normal family history can help guide us. “ ” When collecting family history, it is important to set expectations
- 16. Record family history so that it can be accessed, updated, and interpreted Joseph 3 y ID, behavioral issues 2 D. 34 y Breast ca High cholesterol 33 y Menopause dx 31 ~60’s Tremor, ? dementia, ataxia El Salvadorian Irish/German FamHx: negative for ID/DD
- 18. Red flags that alert you to genetic risk • Multiple individuals with related conditions • Intellectual disability • Early age at onset • Less often affected sex • Absence of known risk factors • Severe manifestations • Ethnic predisposition • Consanguinity
- 19. What red flags do you see in Joseph’s family history? Joseph 3 y ID, behavioral issues 2 Key Intellectual disability D. 34 y Breast ca High cholesterol 33 y Menopause dx 31 ~60’s Tremor, ? dementia, ataxia El Salvadorian Irish/German Movement disorder Infertility
- 20. Joseph 3 y ID, behavioral issues 2 Key Intellectual disability D. 34 y Breast ca High cholesterol 33 y Menopause dx 31 ~60’s Tremor, ? dementia, ataxia El Salvadorian Irish/German Movement disorder Infertility Joseph’s red flags
- 21. Based on clinical guidelines for intellectual disability testing consider the following diagnoses a) Idiopathic intellectual disability b) Chromosome abnormality c) Micro-duplication/deletion syndrome d) Fragile X syndrome
- 22. How would you focus testing based on family history and clinical guidelines? • Intellectual disability • Early age at onset • Absence of known risk factors Joseph 3 y ID, behavioral issues 2 Key Intellectual disability D. 34 y Breast ca High cholesterol 33 y Menopause dx 31 ~60’s Tremor, ? dementia, ataxia El Salvadorian Irish/German Movement disorder Infertility
- 23. Identify patterns to guide testing Joseph 3 y ID, behavioral issues 2 Key Intellectual disability D. 34 y Breast ca High cholesterol 33 y Menopause dx 31 ~60’s Tremor, ? dementia, ataxia El Salvadorian Irish/German Movement disorder Infertility
- 24. Autosomal Recessive Complex Disease X-Linked Autosomal Dominant Carrier
- 26. Chromosome Abnormality Intellectual disability Intellectual disability ID; Congenital heart defect; Short
- 29. Based on the red flags and patterns what is the most likely diagnosis? Joseph 3 y ID, behavioral issues 2 Key Intellectual disability D. 34 y Breast ca High cholesterol 33 y Menopause dx 31 ~60’s Tremor, ? dementia, ataxia El Salvadorian Irish/German Movement disorder Infertility
- 30. Fragile X Joseph 3 y ID, behavioral issues 2 Key Intellectual disability D. 34 y Breast ca High cholesterol 33 y Menopause dx 31 ~60’s Tremor, ? dementia, ataxia El Salvadorian Irish/German Movement disorder Infertility
- 31. What about other red flags? Joseph 3 y ID, behavioral issues 2 Key Intellectual disability D. 34 y Breast ca High cholesterol 33 y Menopause dx 31 ~60’s Tremor, ? dementia, ataxia El Salvadorian Irish/German Movement disorder Infertility
- 32. What does this mean? What does this mean? What do we do next? Why is this important? Communicate your findings
- 33. What do we communicate about the risk assessment?
- 34. Communication about risk assessment • Explain your findings • Tie together the red flags and patterns • What is Fragile X syndrome • Why evaluating for this diagnosis is important – Joseph – Family members • Next steps in evaluation
- 35. Next steps • Order Fragile X genetic testing • Refer or consult with genetics
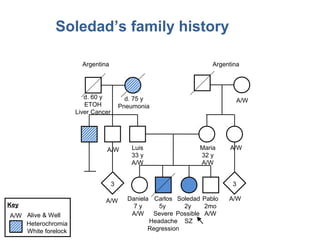
- 37. Soledad’s family history 3 3 Argentina Argentina d. 60 y ETOH Liver Cancer d. 75 y Pneumonia A/W A/W Luis 33 y A/W Maria 32 y A/W A/W Daniela 7 y A/W Carlos 5y Severe Headache Regression Soledad 2y Possible SZ Pablo 2mo A/W A/WA/W Heterochromia White forelock Key A/W Alive & Well
- 38. What red flags do you see in Soledad’s family history? 3 3 Argentina Argentina d. 60 y ETOH Liver Cancer d. 75 y Pneumonia A/W A/W Luis 33 y A/W Maria 32 y A/W A/W Daniela 7 y A/W Carlos 5y Severe Headache Regression Soledad 2y Possible SZ Pablo 2mo A/W A/WA/W Heterochromia White forelock Key A/W Alive & Well
- 39. Red Flags 3 3 Argentina Argentina d. 60 y ETOH Liver Cancer d. 75 y Pneumonia A/W A/W Luis 33 y A/W Maria 32 y A/W A/W Daniela 7 y A/W Carlos 5y Severe Headache Regression Soledad 2y Possible SZ Pablo 2mo A/W A/WA/W Heterochromia White forelock Key A/W Alive & Well
- 40. What inheritance pattern do you see in Soledad’s family history? 3 3 Argentina Argentina d. 60 y ETOH Liver Cancer d. 75 y Pneumonia A/W A/W Luis 33 y A/W Maria 32 y A/W A/W Daniela 7 y A/W Carlos 5y Severe Headache Regression Soledad 2y Possible SZ Pablo 2mo A/W A/WA/W Heterochromia White forelock Key A/W Alive & Well
- 41. Autosomal recessive Luis 33 y A/W Maria 32 y A/W Daniela 7 y A/W Carlos 5y 1mo Severe Headache Regression Soledad 2y 3mo Possible SZ
- 42. Also, autosomal dominant d. 75 y Pneumonia Heterochromia White forelock
- 43. How would you communicate about family history collection and interpretation? What does this mean? What does this mean? What do we do next? Why is this important?
- 44. Communicate • Why you are collecting the information and how it can be used • That collecting family history information is an ongoing process • Informative questions • What your finding mean for the patient • Risks to family members
- 45. Communicate in a patient centered way • Ask about family’s perceptions of condition and risk • Be sensitive to family reactions and emotions • Put risk into perspective
- 46. Next steps • Create differential diagnosis • Communicate interpretation and suspected diagnosis • Genetic testing • Refer or consult with genetics
- 47. Risk assessment for complex diseases
- 48. Key Points in Genomic Risk Assessment 1. Collect history that indicates family structure and manifestations of disease 2. Assess patterns and red flags 3. Use risk to adapt plan for genetic testing, management, counseling etc. 4. Communicate with families about risk in a patient centered way
- 49. Homework/Practice • Practice identifying red flags & inheritance patterns (Cardiomyopathy Case) • High comfort level: Practice recurrence risk counseling (Spinal Muscular Atrophy & Duchenne Muscular Dystrophy) Find the homework at http://www.nchpeg.org/neuro/
- 50. Thank you! [facilitator contact information]
Editor's Notes
- #2: Facilitator Script: [ See supplementary information for example introduction.]
- #4: Key Point: Neurologic disorders are complex diseases influenced by both genes and environment. Facilitator Script: We will begin with some very brief background about genomic contribution to neurologic disease. We know that n eurologic disorders are complex diseases influenced by both genes and environment. What does that mean? Different conditions have varying degrees of genetic vs. environment factors contributing to disease development The etiology of some conditions are due almost entirely to genetic variants (these may be chromosomal or single gene). Most individuals with the genetic variant will develop the condition and environment plays a small role. Many rare disorders fall into this category. Most common conditions fall in the categories of “multifactorial inheritance,” or complex inheritance, where both genetic variants and the environment play a role to a varying degree.
- #5: Key Point: The genomic contribution to neurologic disease runs the spectrum from single gene-high penetrance to multiple genes-low penetrance. Facilitator Script: Additionally, genes have different “strength” in influencing phenotype. The genomic contribution to neurologic disease runs the spectrum from single gene-high penetrance to multiple genes-low penetrance. Some neurologic disorders are Mendelian and caused by changes in a single gene. Most of them are rare disorders. Many neurologic disorders have multiple more common gene variants with modest or moderate effect conferring a risk for the disease, which may or may not result in disease (reduced penetrance). Reduced penetrance is when you have the genetic predisposition for disease but do not go on to develop the disease.
- #6: Key Point: Neurological diseases with a single gene etiology are inherited in all patterns. Facilitator Script: To determine whether there could be a genetic etiology for a patient ’s presentation, we can look for patterns in the family. Neurological diseases with a single gene etiology are inherited in all patterns. Here we see example inheritance patterns of autosomal dominant and X-linked recessive. AD disease typically seen in every generation but there are exceptions—small families, reduced penetrance 50% chance to pass on condition. X-linked mutation in gene on X chromosome; males have worse symptoms than females. Females may be silent carriers, or have mild symptoms, depending on the condition. Carrier female has 50% chance to pass on X chromosome with mutation to children. In addition to these two examples, we know that neurologic disease can be inherited in other patterns, including autosomal recessive, complex, and mitochondrial patterns.
- #7: Key Point: Genomic Risk assessment helps inform diagnosis and decisions about diagnostic testing and patient management. Facilitator Script: To uncover these genomic contributions that are influencing our patients ’ health, we have to go beyond the patient in front of us and look also at what is going on in the patient ’ s family. We will be exploring and practicing the process of genomic risk assessment today. Genomic risk assessment helps inform diagnosis and decisions about diagnostic testing and patient management , as well as informing about risks for other family members.
- #8: Facilitator Script: In this workshop, we will cover these topics:
- #9: Key Point: To get at the genomic information we need to add detail to the information a patient gives us at intake. Facilitator Script: To get at the genomic information we need to add detail to the information a patient gives us at intake. We need to ask the right questions and obtain enough detail to: Identify genetic red flags in the personal and medical history Recognize inheritance patterns that can help in determining a differential and informing risks to relatives
- #10: Key Point: We need to ask questions about how the disease manifests, and in whom, and how are they related to your patient. Facilitator Script: We need to ask questions about how the disease manifests, and in whom, and how are they related to your patient. Aim is to clarify: Structure of the family Nature of relationships How a given disease is manifesting in the family
- #11: -TRANSITION- Facilitator Script Let’ s walk through this with a model case. Here is Joseph, he is a three-year-old male referred by his pediatrician for developmental evaluation. Upon starting preschool, he was noted to be significantly delayed in comparison to his peers. In the notes from the pediatrician, the family history was recorded to be negative for ID/DD. Image: http://www.pediatricsconsultantlive.com/image/image_gallery?img_id=1894519&t=1309461487023
- #12: Facilitator Script: Upon intake, you collect the following information. Developmental history has been predominately normal, as you can see here. Of note, he has speech delay, trouble dressing himself, and some behavioral problems. MORE DETAIL (if asked by participants): Birth History: Normal (Joseph was the product of a reportedly normal pregnancy and delivery. Patient was born via a normal spontaneous vaginal delivery at 40 weeks gestation and weighed 9 pounds, 3 ounces. His neonatal course was uneventful. Mother believes newborn screening was done and the results were normal) Developmental History: hearing normal gross motor milestones on time sat at 7 months, walked at 13 months currently walks and runs well cannot dress himself speech delays spoke first words at 28 months (four, two-word phrases ) behavioral problems, such as hyperactivity and temper tantrums Social History: Joseph lives with his parents and his older sister both English and Spanish are spoken in the home Past Medical History: unremarkable
- #13: -INSTRUCTIONS- Large group discussion to assess learners’ prior understanding of family history interviewing. Jot down ideas generated by participants on white board/iPad/paper easel. Facilitator Script : This is the information Joseph’s parent have filled out on the intake form at your office. What additional questions would you ask about his family history? Additional prompts What follow up questions do we need to ask his parents about their family history? What are you concerned about? What else do you want to know? What else do you want to know about the individuals identified with conditions here? Note : If people suggest genetic testing/Fragile X, refocus on the family history. For example: “We will get to testing, but right now let’s focus on the family history.”
- #14: Key Point: Pedigrees are one option for family history collection that provide a rapid, concise way to capture family structure and disease patterns. -INSTRUCTIONS- Use video to demonstrate a concise targeted interview that builds on an intake form and pedigree drawing. Handouts available for participants on pedigree symbols, resources for assistance, and inheritance patterns. This is for demonstration purposes only. Facilitator Script: Let’s look at one way to uncover this information quickly. -CLICK ON VIDEO TO LAUNCH- Script after video: This showed a model for how family history collection could go. Pedigrees are one option for family history collection that provide a rapid, concise way to capture family structure and disease patterns. As we will talk about in a few minutes, an advantage to using a pedigree for family history documentation is that it can help reveal patterns in the family history. Think about: If this video uncovered the things we wanted to know about the family history? And if there were any things revealed that we had not thought to ask about?
- #15: Key Point: Family history questions should include the details on this list -INSTRUCTIONS- Debrief from the video, focusing on general types of information that was collected. Facilitator Script: From the demonstration, we saw the items on this generalized list being collected by the physician. Some general pointers to keep in mind: We want to identify the family structure Are relationships full, half, step? Need to ask about unaffected relatives - important for accurate risk calculations Need to ask about both maternal and paternal relatives. Third degree relatives (cousins) can be critical for some conditions. We want to know about the presence and extent of disease – who has disease and the brief clinical history of those affected Ages of onset and death Medical conditions and Chronic illnesses Anyone with a history of infertility, multiple miscarriage, or stillbirth Intellectual disability or autism Congenital birth defects It is important to get specific details if anyone has any of these conditions, because they can be associated with some syndromes. Don’ t worry about memorizing this list – see provided point-of-care tool to help interview, interpret and classify risk for your patient. -HANDOUT AVAILABLE-
- #16: Key Point: Communication regarding both the collection and interpretation with the family can help to elicit accurate and detailed information and aid in understanding and patient acceptance. Facilitator Script: When collecting family history, it is important to set expectations. In addition to the specifics of family history collection, we also heard modeling for communication with families about the importance of family health history. Communication regarding both the collection and interpretation of family history with the family can help to elicit accurate and detailed information and aid in understanding and patient acceptance. You often have long-term relationships with your patients, and families will often come back with additional family history information if they know this is important for their child ’s diagnosis.
- #17: Key Point: It is important to not only collect the information but also record it in a way that other health professionals can readily access, update, and interpret the information. Facilitator Script When families do provide new or updated family history information at an updated appointment, having family history documented from the initial work-up can be helpful for you or other healthcare providers to access. Pedigrees can be especially useful for this. Think back to the records that came with the referral for Joseph. The family history was noted to be “unremarkable for ID/DD” There were no other cases of ID, but we did identify a number of conditions in the family that are notable, Which may help us come to a diagnosis for Joseph It is important to not only collect family history information but also record it in a way that other health professionals can readily access, update, and interpret the information for continued patient management .
- #18: Key Point: Red flags are disease characteristics that indicate increased genetic contribution to disease (genetic predisposition). Facilitator Script : With a detailed family history now we can identify “red flags” Red flags are disease characteristics that indicate increased genetic contribution to disease.
- #19: Key Point: Red flags represent increased genetic load or predisposition. -INSTRUCTIONS- Continue to debrief on the video: Discuss how/why each red flag indicates increased risk For time purposes, you may just discuss a few red flags, as suggested below. Facilitator Script : Here are some of the red flags that would alter you to genetic risk. Multiple individuals with the same or related conditions Multiple affected relatives suggests a shared risk factor, especially for unusual conditions Also, the relationship: Closer the relationship, the more genes are shared Less often affected sex: For example, breast cancer in a male or autism in a female Absence of known risk factors: For example, ID without risk factors or type II diabetes in a fit, young person with healthy diet. Consanguinity: Close biological relationship between parents. Shared ancestry increases the chance of inheriting an ancestors gene mutation. However, just because you identify a red flag does not mean that we are dealing with a single gene condition (identifying red flags in a family does not diagnosis a single-gene disorder). And, not having many red flags in the family does not mean the condition does not have a genetic component. DESCRIPTION OF OTHER FLAGS: ID or autism Early age of onset: Genetic forms of a disease tend to occur earlier than sporadic forms of the same disease Severe manifestations: multiple congenital anomalies, bilateral tumors, or rapid progression Ethnicity: certain genetic diseases are more frequent in some populations -HANDOUT AVAILABLE -
- #20: -INSTRUCTIONS- LARGE GROUP DISCUSSION to assess prior understanding of the concept of red flags. Jot down participant responses on a white board, paper easel, or tablet. Compare list to those identified on following slide. Facilitator Script : What red flags do you see in Joseph’ s family history? What clues suggest an increased risk for genetic disorder in the family? ADDITIONAL PROBES If early age of onset is named, asked who are you concerned about with early age of onset? What about this maternal uncle with tremor, dementia, and ataxia symptoms? Is this an unusual combination of clinical features?
- #21: Key point: Joseph’ s family demonstrates earlier age of onset, intellectual disability / developmental delays, severe manifestations, and disease in the absence of risk factors. -INSTRUCTIONS- Use this slide to debrief from large group discussion. Facilitator Script: Important red flags in this family include: Earlier age at onset of disease than expected (early menopause in maternal aunt, early breast cancer in paternal grandmother) Developmental delays or intellectual disability (ID) Severe manifestations (tremor-ataxia)
- #22: Facilitator Script: We have identified some red flags in the family, and based on Joseph ’s history of ID alone, there are clinical guidelines that suggest front line testing. Based on the clinical guidelines for children who present with ID, we would consider evaluation for the following diagnoses:
- #23: Facilitator Script: You could investigate all of those conditions at once, or we can look to the family history to help prioritize our differential and begin with the most likely diagnosis. How would you focus testing based the clinical guidelines AND the family history?
- #24: Facilitator Script: We can look for patterns in the family to help guide testing. These red flags provide are just one part of the picture. We also need to look at inheritance patterns to narrow our differential, guide testing, and identify risk for family members. -HANDOUT AVAILABLE-
- #25: Key point: Inheritance patterns can inform your differential diagnosis and testing strategy. Facilitator Script : Let’s see if we can identify an inheritance pattern in Joseph’s family history. Here are just some of the inheritance patterns that can be seen with neurogenetic conditions: AR, XL, AD, and complex disease. We will be looking at some of these patterns in greater detail as we look for patterns that could be associated with the conditions on our -MORE DETAILS- Autosomal Recessive Inheritance Typically in one individual or individuals in same sibship/generation Parents of an affected individual are obligate carriers of a mutation The chance two carrier parents will have an (another) affected child is 1/4 (25%) Autosomal dominant inheritance Affected individuals in every generation Any child of an affected individual has a 50% chance of inheriting the trait; unaffected family members do not pass it to their children Males and females equally likely to be affected (although there may be sex-limited expression, such as with ovarian cancer) X-linked inheritance Males more often or more severely affected Heterozygous females are typically unaffected but may express trait with variable severity (depending on X inactivation) share clinical example to emphasize changing definitions of recessive disease: DMD cardiomyopathy, fabry etc. Mutations are transmitted from an affected male to all his daughters; mutations are transmitted through carrier females Carrier females have a 50% chance of passing the condition to their sons Complex disorders disorders that result from complex interactions between multiple predisposing factors (ex: changes at multiple genetic loci) AND environmental exposures (such as similar behaviors) Inheritance does not follow that of typical single-gene conditions May be seen as familial clustering due to shared genes and environmental factors Risk of recurrence/occurrence is higher if more affected individuals Risks of disease to other relatives is based on empiric data -HANDOUT AVAILABLE-
- #26: Facilitator Script: The first on our list is idiopathic ID Even after extensive evaluation, most cases of intellectual disability are idiopathic, with No identifiable cause This would most likely be isolated but might see clustering in the family in a complex inheritance pattern.
- #27: Facilitator Script: The second cause of ID on our list is chromosomal abnormalities. This covers the gamut from aneuploidy such as Trisomy 21 to balanced translocations that can be passed down in a family. Often de novo Clinical features are variable: Intellectual disability Minor malformations (e.g., dysmorphic features) Birth defects Short stature Less commonly due to translocations Family members can have same or similar feature Increase in miscarriages infertility and stillbirth
- #28: Facilitator Script: In microdeletion or duplication syndromes, we would also expect to see variable clinical features ID or autism Birth defects Minor malformations (e.g., dysmorphic features) Seizures Most often de novo Can be passed down but can also present with different clinical features Most del/dup are on autos.omal chromosomes that are inherited in AD fashion
- #29: Facilitator Script: Fragile X syndrome Clinical features may but not necessarily include characteristic facies (large jaw and ears, long face), hyperactivity, autistic-like behaviors, and mild to moderate intellectual disability. May see carriers with premature ovarian insufficiency or Fragile X associated tremor ataxia syndrome. X linked inheritance with boys more severely affected than girls. Due to an unstable mutation that can expand, there can be variable expression in diff generation. -NOTE- Additional Fragile X resources for the facilitator are available in the Facilitator Guide.
- #30: -INSTRUCTIONS- Transition to identification of Fragile X in family. Large group discussion/answers Facilitator Script : We have examined the typical inheritance patterns for the conditions on our differential. Based on the red flags and patters that we see in Joseph ’s family, what is the most likely diagnosis?
- #31: Key Point: The Red Flags and Inheritance patterns help point to a diagnosis: Fragile X syndrome Facilitator Script: The most likely cause of Joseph’s delay is Fragile X syndrome. His history of developmental delay and family history shows an X-linked pattern of inheritance among relatives with early-onset infertility and tremor-ataxia symptoms are suspicious for an FMR1 mutation. Fragile X testing is recommended for individuals with: Intellectual disability or autism, Premature ovarian insufficiency, Late onset intention tremor and cerebellar ataxia of unknown origin, or A family history of Fragile X syndrome -HANDOUT ON THE BACKGROUND AND LEARN MORE ON FRAGILE X- -NOTE- Additional Fragile X resources for the facilitator are available in the Facilitator Guide.
- #32: Key Point: Even when collecting a targeted family history, you may identify genetic risks unrelated to the patient’s HPI. Facilitator Script: The most likely diagnosis in this family is Fragile X syndrome, but we need to be aware of the other information that we collected. In this family, the early onset breast cancer is a red flag. This family is at increase risk to have a strong genetic factor causing cancer. Even when collecting a targeted family history, you may identify genetic risks unrelated to the patient’s HPI. These additional risks can be addressed by a genetics professional. In this case, the patient’s father could be referred to cancer genetic counseling due the history of early onset breast cancer in his family. TAKE AWAY includes more information about how to find a genetics professional, the roles of a genetics professional, and the different ways you can collaborate in patient care with these specialists.
- #33: Facilitator Script: Now that we have completed our family history assessment, we need to communicate these findings to the family
- #34: -INSTRUCTIONS- LARGE GROUP DISCUSSION to reflect on how and what to communicate with families about the risk assessment. Jot down participant responses on a white board, paper easel, or tablet. Compare list to those identified on following slide. Facilitator Script: What do you think is important information to convey to the family about your findings? ADDITIONAL PROBES What would you want to tell the family about your risk assessment? What is important for them to know before moving on to the next steps in diagnosis? Note: The participants may go straight to the communication that is part of counseling and informed consent about Fragile X genetic testing. Ask them to take a step back and focus on communication that specifically addresses the interpretation of family history risk assessment. There will be an opportunity to engage in discussion about genetic testing communication in later sessions.
- #35: Key Point: Communication regarding the family history assessment findings can aid in family understanding and compliance, and promotes shared decision making. Facilitator Script: Communication regarding the family history assessment findings can aid in family understanding and compliance, and promotes shared decision making. We want to explain the findings in a patient or family centered way, including why we think this is Fragile X syndrome Introduce the idea that multiple different medical conditions in a family can be tied together with a single underlying cause, such as Fragile X syndrome We can tie together the pattern of ID, infertility, and movement disorder as a possible presentation of Fragile X syndrome. We can share some brief information about Fragile X syndrome, which may include some patient education materials and/or appropriate websites. Explain why the diagnosis is important for both Joseph ’s management as well as for other family members. Diagnosis can help to understand Joseph ’s condition, identify services Diagnosis may be beneficial to the management of Joseph ’s uncle Recurrence risk for other family members – carrier women have 50% chance to have a child with Fragile X Discuss the next steps in evaluation
- #36: Key Point: An individual ’s level of comfort with genomic evaluation determines if genetic testing and/or referral to a genetics professional is the best next step. Facilitator Script: Based on the suspected diagnosis of Fragile X syndrome, next steps would include: Ordering Fragile X testing And possibly referring or consulting with genetics Everyone has a different comfort level with genetic risk assessment and genetic conditions vary widely in their complexity. Consider consulting with genetic specialist when you have questions about the interpretation of the family history, or for further assessment. A geneticist or genetic counselor can: 1) Provide comprehensive family history risk assessment, both for Fragile X and other conditions in the family; 2) Explore pros and cons of genetic testing for Fragile X syndrome, including psychosocial counseling; 3) Explain the risks to other family members and future children; 4) Help to arrange Fragile X testing through Joseph’s insurance company; and 5) Provide contact information to advocacy and support groups.
- #37: -INSTRUCTIONS- Signal of transition to small group practice. Make sure everyone has the toolkit and Soledad handout to complete the activity. Facilitator Script Now, you all will have an opportunity to practice risk assessment for another patient, Soledad. Break into groups of 3 – 5 and work together on the case for about 10 minutes. Read through Soledad ’s history and then work through the discussion questions with your group. Use the toolkit to help you identify red flags, inheritance patterns, and communication strategies. Identify one person to be spokesperson for the group. After you discuss as a small group we will reconvene and discuss your findings with the larger group. -INSTRUCTIONS- Small group work for about 10 min, or as your time allows. Walk around the room to hear what the groups are saying. Pull up the pedigree on the following slide after a few minutes so that the participants to not spend too much time trying to draw a pedigree (not the goal of the activity).
- #38: -INSTRUCTIONS- Show this pedigree after a few minutes of small group work.
- #39: -INSTRUCTIONS- LARGE GROUP DISCUSSION to assess prior understanding of the concept of red flags. Jot down participant responses on a white board, paper easel, or tablet. Compare list to those identified on following slide. Facilitator Script What red flags do you see in Soledad’ s family history? What clues do you see that suggest an increased risk for genetic disorder in the family? ADDITIONAL PROMPTS IF NEEDED Multiple relatives with the same or similar questions? (Carols and Soledad, paternal relatives) Disease in the absence of risk factors? (Carlos) Severe manifestations? (Carlos and Soledad)
- #40: Facilitator Script: Red Flags include: Multiple affected (close) relatives: Carlos and Soledad; paternal relatives with hereochromia and white forelock Disease in the absence of risk factors Severe manifestations
- #41: -INSTRUCTIONS- LARGE GROUP DISCUSSION to assess prior understanding of inheritance patterns. Participants will call out inheritance patterns (there are two).
- #42: Facilitator Script: This family history is suggestive of autosomal recessive inheritance. We cannot say for sure, but this is the most likely inheritance pattern based on the history. We would suspect AR inheritance in cases where two or more siblings are affected with unaffected parents, or individuals in the same generation (like cousins) are affected with unaffected cousins. Additionally, multiple children in the same generation with early childhood death is suggestive of AR inheritance. The parents would be obligate carriers. The chance two carrier parents will have an (another) affected child is 1/4 (25%)
- #43: Facilitator Script: We also see what could be autosomal dominant inheritance in the paternal side with this hearing loss syndrome. In AD inheritance we typically see: Affected individuals in every generation Although, many conditions have reduced penetrance which can make the family history pattern difficult to interpret Males and females equally likely to be affected (although there may be sex-limited expression, such as with ovarian cancer) Any child of an affected individual has a 50% chance of inheriting the trait; unaffected family members do not pass it to their children This condition is called Waardenburg syndrome, and you may have seen pictures of this condition in textbooks. Individuals with Waardenburg have hearing loss, sometimes different colored eyes (heterochromia), pale eyes, and a white forelock or early graying of the hair. As in Joseph ’s family history with his grandmother with a possible genetic cause of breast cancer, we see another genetic condition running through Soledad’s family. This history is unrelated to Soledad ’s and Carol’s presentation, but it is helpful to identify other genetic conditions that may be in the family, and rule out a relationship to your patient’s conditions.
- #44: -INSTRUCTIONS- LARGE GROUP DISCUSSION to assess prior understanding of communication strategies. Participants will discuss the three topics below. Elicit conversation for each question. Facilitator Script: What would you communicate about the family history collection process? What facts would you communicate about your findings in the family history? What are some ways you would you ensure your communication was patient centered?
- #45: Key Point: Communication regarding both the collection and interpretation with the family can help to elicit accurate and detailed information and aid in understanding and patient acceptance. Script: It is important to talk with the family about: Expectations and why FHx is important Updating FHx as they learn more information It is important to ask the right questions to elicit the FHx data that will be most helpful in your assessment After FHx assessment, it is important to communicate with families about the assessment and next steps In addition to moving towards a diagnosis, we can also think about risk for other families members. In this case, we are already suspicious of an autosomal recessive conditions. AR conditions convey a 25%, 1 in 4, recurrence risk for future children. This would be very important to communicate if the parents are considering having other children. You can explore and practice these kinds of recurrence risk calculations and communications in the homework activities available to you.
- #46: Facilitator Script: What are some ways you would you ensure your communication was patient centered? Ask about family ’s perceptions of condition and risk Be sensitive to family reactions and emotions Put risk into perspective
- #47: Facilitator Script: The next steps for this case include…
- #48: Key Point: Risk assessment for complex, or common, conditions should consider a combined effect of genetic contribution to disease development and contributions from environmental, developmental, and lifestyle factors. -INSTRUCTIONS- Transition from case to summary and wrap-up. Facilitator Script: We have just gone through an application of risk assessment for children for whom we identified a strong suspicion of a single gene disorder. Often the children you see in clinic are not going to have a single syndrome identified for their disease rather it will be a common complex condition with moderate genetic contributions. So we need to think about risk assessment for both single gene and complex conditions. Risk assessment for complex, or common, conditions should consider a combined effect of genetic contribution to disease development and contributions from environmental, developmental, and lifestyle factors.
- #49: Facilitator Script: In summary, we have explored these key points in risk assessment…
- #50: Facilitator Script: For additional practice of these concepts and some advanced cases, you can go to the NCHPEG website and use the following web-based cases. There is additional practice on identifying red flags and inheritance patterns, Applying family history of a common indication, headaches, for patient management, And for those who have a high comfort level with genomic risk assessment and patient communication, there are two cases on recurrence risk counseling for families with a known diagnosis of SMA and DMD. Genetic counseling for recurrence risk can be complicated and many providers will refer families who are seeking recurrence risk information to a genetics professional. However, there are some neurologists who do counsel such families and these cases can be useful practice. We ’ve talked a little about recurrence risks for autosomal recessive and dominant and X-linked conditions. SMA and DMD are relatively common conditions seen in the neurology clinic, but there are some nuances to the risk assessment and recurrent risk counseling, so we provide these are more advanced practice.
















































![Thank you!
[facilitator contact information]](https://image.slidesharecdn.com/riskv2-001-15-13final-130506112312-phpapp01/85/Session-One-Risk-Assessment-50-320.jpg)