01 - Introduction and Review - Wade 7th
- 1. Chapter 1 1 Chapter 1 © 2010, Prentice Hall Organic Chemistry, 7th Edition L. G. Wade, Jr. Introduction and Review
- 2. Chapter 1 2 Organic Chemistry • Organic chemistry is the chemistry of carbon compounds. H5C6 N H O O C6H5 OH O O O O OH O O O CH3 O O H5C6 HO Taxol O CH3O H NCH3 HO Codeine 2-Pentenylpenicillin N S CHCH2CONH H H O CH3 CH3 COONa CH3CH2CH
- 3. Chapter 1 3 Electronic Structure of the Atom • An atom has a dense, positively charged nucleus surrounded by a cloud of electrons. • The electron density is highest at the nucleus and drops off exponentially with increasing distance from the nucleus in any direction.
- 4. Chapter 1 4 The 2p Orbitals • There are three 2p orbitals, oriented at right angles to each other. • Each p orbital consists of two lobes. • Each is labeled according to its orientation along the x, y, or z axis.
- 5. Chapter 1 5 Isotopes • Isotopes are atoms with the same number of protons but different number of neutrons. • Mass number is the sum of the protons and neutrons in an atom. C 12 6 6C 14
- 6. Chapter 1 6 Electronic Configurations of Atoms • Valence electrons are electrons on the outermost shell of the atom.
- 7. Chapter 1 7 Electronic Configurations • The aufbau principle states to fill the lowest energy orbitals first. • Hund’s rule states that when there are two or more orbitals of the same energy (degenerate), electrons will go into different orbitals rather than pairing up in the same orbital. Electronic configuration of carbon
- 8. Chapter 1 8 Ionic Bonding • To obtain a noble gas configuration (a full valence shell), atoms may transfer electrons from one atom to another. • The atoms, now bearing opposite charges, stay together by electrostatic attraction.
- 9. Chapter 1 9 Covalent Bonding • Electrons are shared between the atoms to complete the octet. • When the electrons are shared evenly the bond is said to be nonpolar or pure covalent. • When electrons are not shared evenly between the atoms, the resulting bond will be polar.
- 10. Chapter 1 10 CHCH44 NHNH33 HH22O ClO Cl22 Lewis Structures C H H H H N H H H O HH ClCl Carbon: 4 e 4 H@1 e ea: 4 e 8 e Nitrogen: 5 e 3 H@1 e ea: 3 e 8 e Oxygen: 6 e 2 H@1 e ea: 2 e 8 e 2 Cl @7 e ea: 14 e
- 11. Chapter 1 11 Double and Triple Bonds
- 12. Chapter 1 12 Bonding Patterns ValenceValence electronselectrons # Bonds# Bonds # Lone Pair# Lone Pair ElectronsElectrons CC NN OO HalidesHalides (F, Cl, Br, I)(F, Cl, Br, I) 44 44 00 55 33 11 66 22 22 77 11 33
- 13. Chapter 1 13 Lone Pairs
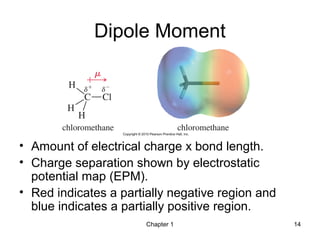
- 14. Chapter 1 14 Dipole Moment • Amount of electrical charge x bond length. • Charge separation shown by electrostatic potential map (EPM). • Red indicates a partially negative region and blue indicates a partially positive region.
- 15. Chapter 1 15 Electronegativity and Bond Polarity • Electronegativities can be used to predict whether a bond will be polar. • Since the electronegativity of carbon and hydrogen are similar, C—H bonds are considered to be nonpolar.
- 16. Chapter 1 16 Charged Species H3O+ NO+ HCO3 - OH H H Formal charge = number of valence electrons – (Formal charge = number of valence electrons – (ee in lone pairs + # bonds)in lone pairs + # bonds) C O OHO N O 6 – (2 + 3) = +1 6 – (2 + 3) = +1 5 – (2 + 3) = 0 6 – (6 + 1) = -1 ++ ++
- 17. Chapter 1 17 Compute the formal charge (FC) on each atom in H3N – BH3. Solved Problem 1 Solution
- 18. Chapter 1 18 Common Bonding Patterns
- 19. Chapter 1 19 Resonance Forms • In a resonance form, only the electrons are moved. Connectivity between atoms stay the same. • The real structure is a hybrid of the different resonance forms. • Arrows connecting resonance forms are double headed. • Spreading the charges over two or more atoms stabilize the ion.
- 20. Chapter 1 20 Resonance Forms Resonance Forms can be compared using the following criteria, beginning with the most important: – Has as many octets as possible. – Has as many bonds as possible. – Has the negative charge on the most electronegative atom. – Has as little charge separation as possible.
- 21. Chapter 1 21 Major and Minor Contributors • The major contributor is the one in which all the atoms have a complete octet of electrons. C O H H C O H H The carbon atom does not have a complete octet of electrons. MAJOR MINOR
- 22. Chapter 1 22 Major and Minor Contributors (Continued) • When both resonance forms obey the octet rule, the major contributor is the one with the negative charge on the most electronegative atom. N C O N C O MAJOR MINOR The oxygen is more electronegative, so it should have the negative charge.
- 23. Chapter 1 23 • Opposite charges should be on adjacent atoms. Non-Equivalent Resonance CH3 O N O CH3 O N O The most stable one is the one with the smallest separation of oppositely charged atoms. MAJOR MINOR
- 24. Solved Problem 2 Chapter 1 24 Draw the important resonance forms for [CH3OCH2]+ . Indicate which structure is major and minor contributor or whether they would have the same energy. The first (minor) structure has a carbon atom with only six electrons around it. The second (major) structure has octets on all atoms and an additional bond. Solution
- 25. Solved Problem 3 Chapter 1 25 Draw the resonance structure the compound below. Indicate which structure is major and minor contributor or whether they would have the same energy. Both of these structures have octets on oxygen and both carbon atoms, and they have the same number of bonds. The first structure has the negative charge on carbon; the second has it on oxygen. Oxygen is the more electronegative element, so the second structure is the major contributor. Solution
- 26. Chapter 1 26 Resonance Forms • The structure of some compounds are not adequately represented by a single Lewis structure. • Resonance forms are Lewis structures that can be interconverted by moving electrons only. • The true structure will be a hybrid between the contributing resonance forms.
- 27. Chapter 1 27 Resonance Forms for the Acetate Ion • When acetic acid loses a proton, the resulting acetate ion has a negative charge delocalized over both of the oxygen atoms. • Each oxygen atom bears half of the negative charge, and this delocalization stabilizes the ion. • Each of the carbon–oxygen bonds is halfway between a single bond and a double bond, and they are said to have a bond order of 1½.
- 28. Chapter 1 28 Structures and Formulas Extended Condensed CH3CH3H C C H H H H H H C C C H H H H C H H H H H 1 2 3 41 2 3 4 1 21 2 CH3CH2CH2CH3
- 29. Chapter 1 29 Structures and Formulas Extended Condensed H C C C H H H H C Br H H H H CC C H H HC H H OH OH H 1 2 3 41 2 3 4 1 2 3 41 2 3 4 CH3CH2CHCH3 Br HOCH2CH2CCH3 O
- 30. Line-Angle Formulas Chapter 1 30 H C C C H H H H C H H H H H C C C C H C C C H H H H C H C H H H H H C O H O H 1 2 3 4 1 2 3 4 5 6 1 2 3 4 5 6
- 31. Chapter 1 31 Line-Angle Formulas (Continued)
- 34. Chapter 1 34 Calculating Empirical Formulas The following are items that need to be considered when calculating empirical formulas: – Given % composition for each element, assume 100 grams. – Convert the grams of each element to moles. – Divide by the smallest moles to get ratio. – Molecular formula may be a multiple of the empirical formula.
- 35. Chapter 1 35 Arrhenius Acids • Arrhenius acids are substances that dissociate in water to give H3O+ ions.
- 36. Chapter 1 36 Arrhenius Bases • Arrhenius bases are substances that dissociate in water to give hydroxide ions.
- 37. Chapter 1 37 Brønsted-Lowry Acids and Bases Brønsted-Lowry acids are any species that donate a proton. Brønsted-Lowry bases are any species that can accept a proton.
- 38. Chapter 1 38 Conjugate Acids and Bases • Conjugate acid: when a base accepts a proton, it becomes an acid capable of returning that proton. • Conjugate base: when an acid donates its proton, it becomes capable of accepting that proton back.
- 39. Chapter 1 39 Effect of Electronegativity on pKa • As the bond to H becomes more polarized, H becomes more positive and the bond is easier to break.
- 40. Chapter 1 40 Effect of Size on pKa • As size increases, the H is more loosely held and the bond is easier to break. • A larger size also stabilizes the anion.
- 41. Chapter 1 41 Effect of Resonance on pKa • If the negative charge on an atom can be delocalized over two or more atoms, the acidity of that compound will be greater than when the negative charge cannot be delocalized. • The ethoxide anion is less acidic than the acetate ion simply because the acetate ion can delocalize the negative charge. • Methanesulfonic acid can delocalize the charge in three different resonance forms, making it more acidic than the acetate ion.
- 42. Chapter 1 42 Nucleophiles and Electrophiles • Nucleophile: Donates electrons to a nucleus with an empty orbital. • Electrophile: Accepts a pair of electrons. • When forming a bond, the nucleophile attacks the electrophile, so the arrow goes from negative to positive. • When breaking a bond, the more electronegative atom receives the electrons.
- 43. Chapter 1 43 Nucleophiles and Electrophiles (Continued)
Editor's Notes
- #18: Copyright © 2006 Pearson Prentice Hall, Inc.
- #25: Copyright © 2006 Pearson Prentice Hall, Inc.
- #26: Copyright © 2006 Pearson Prentice Hall, Inc.
- #33: Figure: 01_07-020T1-3.jpg Title: Condensed Formulas for Double and Triple Bonded Compounds Caption: Condensed Structural Formulas for Double and Triple Bonds Notes: In condensed formulas double and triple bonds are drawn as they would be in a Lewis structure showing two dashes for a double bond and three dashes for a triple bond.
- #34: Figure: 01_07-033T1-4.jpg Title: Line-angle Drawings Caption: Examples of Line-angle Drawings Notes: Line-angle formula (a.k.a. skeletal structure or stick figure) is a shorthand way of drawing organic compounds. Bonds between carbons are represented by lines. Carbons are present where the lines begin and end, and where the lines meet. Nitrogen, oxygen, and halogen atoms are shown but hydrogens are not drawn unless they are bonded to a drawn atom.























![Solved Problem 2
Chapter 1 24
Draw the important resonance forms for [CH3OCH2]+
. Indicate which structure is major and minor
contributor or whether they would have the same energy.
The first (minor) structure has a carbon atom with only six electrons around it. The second (major)
structure has octets on all atoms and an additional bond.
Solution](https://image.slidesharecdn.com/chapter01wade7thcgd-140409011227-phpapp01/85/01-Introduction-and-Review-Wade-7th-24-320.jpg)


















