15446183.ppt
- 1. General University Hospital in Prague Metals and Dental Alloys Assoc. Prof. M.D. Ivan Malbohan, Ph.D. M.D. Lenka Fialová , Ph.D. Medical Chemistry and Biochemistry 1st year – Winter term - Dentistry First Faculty of Medicine Institute of Medical Biochemistry and Laboratory Diagnostics © Institute of Medical Biochemistry and Laboratory Diagnostics of the General University Hospital in Prague and of The First Faculty of Medicine of Charles University - 2005-2019
- 2. Stomatological materials Metallic Dental metals and alloys Dental amalgams Nonmetallic Dental plasters Dental cements Dental porcellains (silicates) Dental resins Impression materials Modell materials Modelling materials Grinding and polishing tools and means 2018/2019 2 Metals and Dental Alloys
- 3. Introduction Metals belong to the oldest dental materials. Pure metallic elements are not frequently used in stomatology. As main materials are the pure metals used only for special purposes (titanium – implants), because the properties of the elementary metal are usually not suitable for the needs of clinical practice. Sometimes the pure gold, which is relatively soft, is used for preparation of high quality, but very expensive inlays. 2018/2019 3 Metals and Dental Alloys
- 4. Requirements for the metallic material For preparation of artificial denture, fix or removable, a material with higher durability, is needed, having also appropriate hardness, stiffness and toughness, but also the plasticity and malleability. Very important is also the resistance of the material to corrosion and to wear and tear. The colour of the material is important esthetically . In contrast the good thermal and electric conductivity means in artificial denture rather a disadvantage. 2018/2019 4 Metals and Dental Alloys
- 5. Requirements for the metallic material All this requirements are best fulfilled by SPECIALLY PREPARED STOMATOLOGICAL ALLOYS 2018/2019 5 Metals and Dental Alloys
- 6. Metallic bond Metallic bond is a specific type of chemical bond, formed between the atoms of metals . Atoms of metals tend to form a stable configuration and throw away the weakly bonded electrons and transform to cations. The valence electrons are delocalized over the entire crystal. In fact, metal atoms in a crystal can be imagined as an array of positive ions immersed in a sea of delocalized valence electrons. 2018/2019 6 Metals and Dental Alloys
- 7. Crystallography of metals Cations of metals immersed in a „ sea of valence electrons“, are organized in a crystal lattice. Most of dental metals crystalize in following unit cells: Body centred cubic (Cr, Mo, W) Particles are placed in the corners and in the middle of a unit cell Face centred cubic (Au, Ag, Pt, Pd, Ir, Cu, Co, Ni, Fe) Particles are placed in corners and in the middle of walls of the unit cell Hexagonal close packed (less frequent - Os, Ru, Zn, Ti) 2018/2019 7 Metals and Dental Alloys
- 8. Crystallographic properties of dental metals By crystallographic methods, with the use of microscope, we follow on the fracture or on the cut the lattice parameters and the types of crystallic lattices and mainly the disturbances of crystalline lattice. The arrangement of a crystalline lattice is not completely regular in real metals. According to the size and shape of the crystallographic anomalies of atomic arrangement we recognize different lattice irregularities. Crystal lattice irregularities Dot Linear Planar Volume 2018/2019 8 Metals and Dental Alloys
- 9. Crystallographic properties of dental metals Dot – missing particle or an extra particle vacancy an interstitial atom a small substitutional atom a big substitutional atom Linear (dislocations) falling out of a part of the edge, elimination of the whole line sliding of atoms out of the regular positions in the crystalline lattice Planar Originate e.g. by removing of a part of the plane of atoms or by its addition to the structure Volume The volume disturbances are the fissures and precipitates (islets of different crystalline structure), present in a crystal. 2018/2019 9 Metals and Dental Alloys
- 10. Crystallographic properties of dental metals Amount and character of the crystal lattice disorders have an influence on the mechanical properties of metals and particularly of their alloys. Presence of these disturbances enables the plastic deformation of the metal. Deformation means a change of the form of the lattice which results in a change without formation of fissures. Plastic deformation is a change of the shape, which remains conserved after elimination of the cause of deformation. 2018/2019 10 Metals and Dental Alloys
- 11. Crystallization process Crystallization starts during the transition from liquid to solid state. Solid state of a metal Liquid state of a metal Crystallization 2018/2019 11 Metals and Dental Alloys
- 12. Crystallization process Mechanism of crystallization starts by the origin of stable crystal seeds (nuclei). Nuclei are tiny volumes of a new phase in the liquid phase to which another atoms attach. Crystallization nuclei possess the crystal structure and are oriented any direction. Crystallization nuclei of solid phase originate: spontaneously directly in the liquid phase – homogenous nucleation on present nuclei of foreign phase – heterogenous nucleation 2018/2019 12 Metals and Dental Alloys
- 13. Crystallization process On the crystallization nuclei progressively attach another atoms and a homogenous crystal originates. During the growth the crystal is limited by the adjacent growing crystals, and therefore the shape of the crystal is uneven. Crystals with uneven shape are labelled as grains. Inside of grains are the particles arranged regularly, but the reciprocal positon of grains is random and irregular. On the border of grains the impurities may place and they may be the point, where the corrosion starts. 2018/2019 13 Metals and Dental Alloys
- 14. Crystallization process Number of grains affects properties of the metal. Better mechanical quality have small-grained metals. This structure is correlated with the highest number of nuclei. The number of grains higher than 500 on mm2 and size of grains 30 m and less is required. Finer structure may be achieved by: Faster cooling down Introducing of foreign fine particles into the liquid alloy as heterogeneous nuclei 2018/2019 14 Metals and Dental Alloys
- 15. Crystallization process During fast cooling down more nuclei are formed. The result is a fine-grain structure having better mechanical quality. During slower cooling down coarse-grained structure originates. Fast cooling Slower cooling Fine grain structure BETTER MECHANIC QUALITY (superior stregth) Coarse- grained structure 2018/2019 15 Metals and Dental Alloys
- 16. Crystallization process If the cooling down of the alloy is too fast, the grain grows faster in one direction - primary branch, from which perpendicularly protrude shorter secondary branches. Herringbone-like branched structure is formed – dendrites. Dendrites possess different composition than other parts of the alloy and show a non- homogeneity of the material. Dendritic structure may weaken the mechanical and corrosion resistance of the alloy. Primary branch Secondary branch 2018/2019 16 Metals and Dental Alloys
- 17. Crystallization process Endogenous crystallization The germ crystals arise uniformly in the whole cast Suitable for the dental alloys – fine-grain, homogenous cast Au-Pt alloys Exogenous crystallization The germ crystals form only on the surface of the cast 2018/2019 17 Metals and Dental Alloys
- 18. Crystallization process Crystallization is always accompanied by the contraction, which is most expressed in the centre of the cast, where the solidification takes place at last. The result of contraction are the contraction defects. Au alloys – contraction 1,4 % Common metal alloys 2,3 – 2,7 % 2018/2019 18 Metals and Dental Alloys
- 19. Alloys Alloy is a mixture of a metal with other metals or other elements or compounds, usually in a form of solid solution. Suitable combination of metals allows the achievement of required quality. As the alloying elements we call the elements which even in very small amount significantly improve the characteristic of the alloy. 2018/2019 19 Metals and Dental Alloys
- 20. Alloys of metals mutually soluble in fluid and solid state When the elements contained in the alloy are completely mutually soluble and retain this quality even during solidification, a solid solution results. Characteristic Only one phase exist Not only atoms of the fundamental metal, but also the atoms of the additive element are present in the crystal lattice . Base is the atom lattice of the basic component, with the atoms of the admixed element inside. Depending on position of admixed atoms we differentiate two basic types of mixed crystals. 2018/2019 20 Metals and Dental Alloys
- 21. Alloys of metals mutually soluble in fluid and solid state Substitutional alloys The size of atoms metals forming the alloy must not differ more than 15 %. Atoms of the base metal are in his crystal lattice randomly substituted by the atoms of additive metal. Example: Binary systems Au-Pt, Au-Ag Used in dentistry Interstitial alloys Combination of atoms several fold differing in the size The additive element (e.g. N, C) is placed into the crystal lattice of the base metal (large atoms) 2018/2019 21 Metals and Dental Alloys
- 22. Properties of dental alloys Dental metal (alloy) is characterized by following properties: mechanical physical chemical biological 2018/2019 22 Metals and Dental Alloys
- 23. Mechanical properties of dental alloys Modulus of elasticity (transient deformation) Yield strength (permanent deformation) Tensile strength („breaking point“ of the material) Hardness Traction test 2018/2019 23 Metals and Dental Alloys
- 24. Mechanical properties of dental alloys Modulus of elasticity – transient deformation It is a measure of the bending resistance of an alloy Higher modulus of elasticity lower bending during mechanical load It is important in alloys used in porcelain fused to metal (PFM) systems. Higher modulus of elasticity lower susceptibility to splitting of the ceramics 2018/2019 24 Metals and Dental Alloys
- 25. Mechanical properties of dental alloys Yield strength - permanent deformation Quotes the force which causes a permanent deformation of the material (usually 0,1 % or 0,2 %). Higher yield strength higher resistance to stress Low value of yield strength easy deformation of the material Evaluation of the alloys according to the yield strength. 2018/2019 25 Metals and Dental Alloys
- 26. Mechanical properties of dental alloys Firmness of the material Firmness in traction Is characterized as maximal traction, which material endures without breaking Firmness in pressure Is characterized by a pressure, which the material endures without a damage 2018/2019 26 Metals and Dental Alloys
- 27. Mechanical properties of dental alloys Hardness Indicates the ability of an alloy to resist the local stress during the bite Requirements Hardness of prosthetic alloys should not exceed the hardness of the enamel and should be between 125 kg/mm2 340 kg/mm2 (=hardness of the enamel) Sufficient resistance of the material against mastication load Must not damage the teeth in opposite jaw x 2018/2019 27 Metals and Dental Alloys
- 28. Mechanical properties of dental alloys Testing of hardness Hardness according to Vickers Impression of qadrilateral diamond pyramid in the studied material Hardness according to Brinell Impression of steel ball in the studied material 2018/2019 28 Metals and Dental Alloys
- 29. Mechanical properties of dental alloys Very hard materials are usually quite fragile and may break or chip off by impact or by higher strain on the prostheses. For that reasons the too hard materials are unsuitable for the use in stomatology. Hard metals - Ni, Fe, Cr, Co 2018/2019 29 Metals and Dental Alloys
- 30. Physical properties of dental alloys Melting and boiling point All metals with the exception of mercury and gallium are solid at normal room temperature. Temperature necessary for the change from the solid state of the metal to the liquid state is called the melting point. The values of the melting point differ substantially in individual metals and alloys. High melting point Very low melting point Ir, Pt, Pd Ga, In, Sn 2018/2019 30 Metals and Dental Alloys
- 31. Physical properties of dental alloys Density The ratio of mass and volume (g/cm3) The highest density have the gold alloys containing also platinum and iridium and a bit lower the gold alloys with the reduced content of gold. The lightest is the titanium and its alloys. The use of alloys of higher density is better for casting. The density has an influence on the final weight of the whole construction. The heaviest pieces of work are from platinum and gold alloys. The density has also an influence on the costs of the used material. 2018/2019 31 Metals and Dental Alloys
- 32. Chemical properties of dental alloys Important chemical properties Corrosion Surface passivity of the alloy 2018/2019 32 Metals and Dental Alloys
- 33. Chemical properties of dental alloys Corrosion Corrosion is a progressive erosion of the material by chemical or physically-chemical reactions with the surrounding environment. During corrosion in the oral cavity the release of ions or of ion complexes from dental alloys occurs. A manifestation of corrosion may be the change of colour. Alloys with high content of Au and Pt are stable. Alloys based on common metals containing Cr form on the surface a corrosion resistant layer of chromic oxide - passivation effect. 2018/2019 33 Metals and Dental Alloys
- 34. Chemical properties of dental alloys Passivation Passivation is a process forming a protective layer on the surface of an metal preventing corrosion. The protecting layer, called passivation layer, is formed mainly by oxides. The passivation layer prevents the release of ions of elements present in the alloy to the oral cavity. 2018/2019 34 Metals and Dental Alloys
- 35. Galvanic currents May arise at close contact of two different metals in a wet environment of the oral cavity (saliva). Galvanic currents come in existence on a base of different electrode potentials of individual metals and alloys and are quoted in A. Pathologic value 5 A. 2018/2019 35 Metals and Dental Alloys
- 36. Biological properties of dental alloys Besides of above mentioned properties is necessary in stomatological alloys study their relation to living tissues. The cytotoxicity of alloys has to be tested usually on tissue cultures of fibroblasts. The direct contact is also tested to determine if the elution of the parts of alloy arises. Also the change of the color of the material during the contact with living tissues must be monitored. 2018/2019 36 Metals and Dental Alloys
- 37. Biological properties of dental alloys Toxicity Allergic reactions Mutagenity and cancerogenity 2018/2019 37 Metals and Dental Alloys
- 38. Biological properties of dental alloys Toxicity General toxicity of ISO dental alloys was not observed. Local toxicity – is usually of small importance. 2018/2019 38 Metals and Dental Alloys
- 39. Biological properties of dental alloys Allergic reaction Metallic alloys represent an foreign material in the organism. They may therefore induce allergic reactions in patients, but also in dental technicians. Local manifestations In the oral cavity (i.e. tongue coating or edema, small blisters, red colour of oral cavity, pain) General manifestations Fatigue, cephalea (headache) Nausea (feeling on vomiting) 2018/2019 39 Metals and Dental Alloys
- 40. Biological properties of dental alloys Very frequent allergens are nickel, cobalt and chromium. Manifestation of allergy may be so called metallic spots. 2018/2019 40 Metals and Dental Alloys
- 41. Biologic properties of dental alloys Mutagenity and cancerogenity of alloys Mutagenic and cancerogenic effect Berylium and cadmium !!! No more used in dental materials Mutagenic effect Some nickel compounds are carcinogenic Nickel is not a mutagen Chromium(VI) is toxic and mutagenic In stomatology chromium(III) is used 2018/2019 41 Metals and Dental Alloys
- 42. Technology of dental alloys processing The lost wax technique of casting In our country it is a most frequently used technique for casting of metallic dental prostheses (inlays, crowns, bridges). The wax model equipped with an inflow system is immersed into the investment material. When the investment material sets hard the wax is burned out and into the obtained mold the liquid alloy is poured, usually with the help of centrifugal force. 2018/2019 42 Metals and Dental Alloys
- 43. Technology of dental alloys processing Procedure: Preparation of the tooth (or teeth) to receive restoration (grinding) Making an impression of prepared tooth Making of gypsum replica which is an exact model of the dental arch, from which individual pars (die(s)) representing the prepared tooth (teeth) are sectioned Making a wax pattern representing the lost tooth structure. Office Laboratory 2018/2019 43 Metals and Dental Alloys
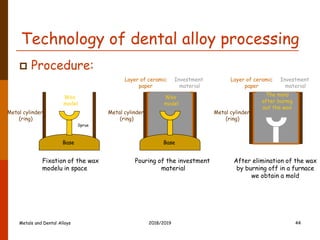
- 44. Technology of dental alloy processing Procedure: Fixation of the wax modelu in space Layer of ceramic paper Investment material Base Metal cylinder (ring) Wax model Base Metal cylinder (ring) Wax model Pouring of the investment material Layer of ceramic paper Investment material Metal cylinder (ring) After elimination of the wax by burning off in a furnace we obtain a mold Sprue The mold after burnig out the wax 2018/2019 44 Metals and Dental Alloys
- 45. Technology of dental alloy processing Procedure: The released cast is cleaned, the sprue is removed from the cast, the cast is finished and polished and than cemented on the prepared tooth or teeth Breaking of the mold Investment material Metal cylinder (ring) Forcing of the molten alloy into the mold usually with the use of centrifugally force. This is necessary to achieve perfect cast of the crown or bridge. Investment material 2018/2019 45 Metals and Dental Alloys
- 46. Classification of dental alloys Dental alloys are divided in three main groups: Alloys with high content of noble metals – high noble alloys Alloys with lower content of noble metals – noble alloys Alloys of base metals – base metal alloys The composition of the alloys must correspond to the relevant ISO (International Standardization Organization ) standards. 2018/2019 46 Metals and Dental Alloys
- 47. Ag Representative parts of noble metal alloys strength hardness Corrosion resistance Cu Pt Pd Zn Ag Fragility of the alloy Antioxidant Strength Hardness Fine-grain Corrosion resistance Fine-grain Harder than gold Au soft 2018/2019 47 Metals and Dental Alloys
- 48. Alloys with high content of noble metals Noble metals ≥ 60 % Au ≥ 40 % Types of „high-noble alloys“ Au-Ag-Pt Au-Cu-Ag-Pd-I Au-Cu-Ag-Pd-II 2018/2019 48 Metals and Dental Alloys
- 49. Alloys with high content of noble metals Au; 78 Au; 76 Au; 56 Ag; 11,5 Ag; 10 Ag; 25 Cu; 10,5 Cu; 11,8 Pt; 9,9 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Au-Ag-Pt Au-Cu-Pd-I Au-Cu-Pd-II Zn Pt Pd Cu Ag Au Yellow Yellow Yellow Au-Cu-Ag-Pd-I Au-Cu-Ag-Pd-II 2018/2019 49 Metals and Dental Alloys
- 50. Alloys with lower content of noble metals Noble metals ≥ 25 % Au content is not specified Types of „ lower content noble alloys“ Au-Cu-Ag-Pd-III Au-Ag-Pd-In Pd-Cu-Ga Ag-Pd 2018/2019 50 Metals and Dental Alloys
- 51. Alloys with lower content of noble metals If the gold content is under 45 % the risk of discolouring a corrosion increases. The gold is usually replaced by palladium. The alloys with lower content of noble metals are adequately strong and hard. 2018/2019 51 Metals and Dental Alloys
- 52. Alloys with lower content of noble metals Au; 40 Au; 20 Ag; 47 Ag; 38,7 Ag; 70 Cu; 7,5 Cu; 10 Pd; 21 Pd; 77 Pd; 25 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Au-Cu-Ag-Pd-III Au-Ag-Pd-In Pd-Cu-Ga Ag-Pd Zn Pd Cu Ag Au Yellow Light Yellow White Hardest White Palladium content higher than 10 % gives to the alloy white color. 2018/2019 52 Metals and Dental Alloys
- 53. Gold dental alloys Gold (Au) is the most stable metal, no oxidation occurs, no change in the oral cavity. Pure gold For the softness and malleability the pure gold is used only exceptionally (inlays, galvanoforms) Alloys 2018/2019 53 Metals and Dental Alloys
- 54. Gold dental alloys Proportional content of gold in an alloy, or the purity, is expressed with the carates or in percents or in thousandths. Carate (c) represents 1/24 of the whole. 24 carates responds to the pure gold. Examples Carates Percents Thousandths Pure gold 24/24 100 1000 Alloy 18 C 18/24 75 750 2018/2019 54 Metals and Dental Alloys
- 55. Gold dental alloys Traditional classification recognizes gold alloys according to hardness. 4 types of gold alloys are recognized: I. soft II. medium III. hard IV. extra hard 2018/2019 55 Metals and Dental Alloys
- 56. Composition of gold alloys 0% 20% 40% 60% 80% 100% I. měkká II. střední III. tvrdá IV. velmi tvrdá Pt/Pd Cu Ag Au Strength Hardness Ductility Corrosion resistance Cu Increases Decreasees Increases 2018/2019 56 Metals and Dental Alloys
- 57. Examples of alloys with high gold content Au 22 CAR Au 18 CAR Pt Low strength alloy Very high strength alloy Au; 91,5 Au; 74,5 Ag; 4 Ag; 8,5 Cu; 4,5 Cu; 11 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Au 22 kar Au 18 kar Pt Pt Pd Cu Ag Au 2018/2019 57 Metals and Dental Alloys
- 58. Examples of alloys with lower gold content Aurosa® Aurix®L Very high strength alloys Au; 20 Au; 65 Ag; 45 Ag; 20 Cu; 15 Cu; 9,6 Pd; 20 Pd; 3 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Aurosa® Aurix®L Zn Pt Pd Cu Ag Au Gold and platinum metals 25 – 75 %. 2018/2019 58 Metals and Dental Alloys
- 59. Base metal alloys The main disadvantage of gold and platinum is very high price. That is why the base metal alloys are used. Chromium – hardness, corrosion resistance Cobalt - firmness and hardness, corrosion resistance Nickel - ductility, malleability, firmness decrease, allergies Molybdenum – hardness Lower density compared with gold alloys. 2018/2019 59 Metals and Dental Alloys
- 60. Base metal alloys Cobalt-chromium alloys Co 53 - 67 % Cr 25 - 32 % Sometimes C is added to increase firmness. Examples of composition Co-Cr-Mo-Si-Mn Co-Cr-Mo-W-Si Co-Cr-Mo-Ti Cr a Mo increase hardness Cobalt alloys are usually stronger and harder nickel alloys. Very hard 2018/2019 60 Metals and Dental Alloys
- 61. Base metal alloys Nickel-chromium alloys Ni 60 – 80 % Cr 10 - 27 % Examples of composition Ni-Cr-Mo-Si Ni-Cu-Mo Higher nickel content increases toxicity (ALLERGIES !!) Very high melting point (1400 – 1600 °C) – difficult casting 2018/2019 61 Metals and Dental Alloys
- 62. Pure titanium from the point of view of mechanical properties titanium is the best dental metal Very resistant to corrosion A passivation layer on the surface is formed very rapidly and after scratching is very fast restored. Biocompatible, light. The alloys are very expensive. Titanium and its alloys 2018/2019 62 Metals and Dental Alloys
- 63. Composition: Cu-Al-Ni-Fe-Zn-Mn; Ag-Sn Many patients do not tolerate Al bronzes. Contraindication: gastric and duodenal ulcers. No ISO standards !! Aluminium bronzes Nice colour • Very low corrosion resistance • Unpleasant metallic taste (high amount of released ions) • Allergic reactions x 2018/2019 63 Metals and Dental Alloys
- 64. Alloys for PFM (porcelain fused to metal) restorations PFM restorations use the benefits of both materials. METAL CONSTRUCTION Mechanic resistance X Low esthetics of alloys FIRED CERAMIC LAYER Convenient esthetic and biological properties X Fragility + 2018/2019 64 Metals and Dental Alloys
- 65. PFM alloys Melting point must be over 1000 °C, because firing of ceramic material takes place at 900 °C. Harmonizing of thermal expandability of metal and ceramic is necessary to prevent separation of both materials during firing. 2018/2019 65 Metals and Dental Alloys
- 66. PFM alloys Materials used in Czech republic Palladium base: Safibond: Au-Ag-Pd-Sn-In-Ga- Zn-Ru Base metals: Oralium Ceramic ( cobalt and chromium) Wiron 99 (chromium and nickel) 2018/2019 66 Metals and Dental Alloys
- 67. Materials for dental implants Metallic At present almost exclusively the materials on base titanium are used. They possess of high strength and resistance to corrosion. Both it is virtue of their very tight hexagonal crystal lattice. Excellent biocompatibility is a result of the very stabile layer of oxides on the surface of the metal (passivation layer). Strength property of titanium is outstanding. (It is also used for the rotaries of the highest-ratings ultracentrifuges !!). Special, more strength alloys also exist, but they are very expensive. 2018/2019 67 Metals and Dental Alloys
- 68. Materials for dental implants Ceramic Ceramic materials are fully oxidized, and therefore chemically very stable. Solely ceramic implants may be used, or the ceramic materials may be used for coating of metallic materials. So called bioactive ceramic materials, which react with the bone and merge together are introduced now. They contain oxyapatite a fluoroapatite [Ca10(PO4)6)O,F2], -wollastonite (SiO2-CaO) in MgO-CaO-SiO2 glass matrix. Further advantage is low thermal and electrical conductivity and similar elasticity as the bone. In the literature they are usually labeled as CPC (calcium phosphate ceramics). 2018/2019 68 Metals and Dental Alloys
- 69. Stomatological solders Solders are special alloys, serving for joining of metallic parts together. Soldering is a process, when the metals are joined together at lower temperature (to 425 °C), when higher temperature is used it is technically brazing. The stomatological prosthetics the term soldering is used even when higher temperatures are used. During soldering different soldering pastes are used, which clear the surface of the alloy from oxides, which would impede the correct joining of the soldered parts. The mechanical cleaning must of course precede. 2018/2019 69 Metals and Dental Alloys
- 70. Stomatological solders The solder must have the melting point lower than the joined material. This means also the different composition, which increases the risk of corrosion in oral cavity. It follows, that mainly the solders on the base of gold or silver are used, to which tin is added (tin lowers the melting point). Solders on the base of gold are used mainly for joining of casts of fix and removable prostheses. Solders on the base of silver are used mainly in orthodontic applications, which stay in the oral cavity for limited time, because silver shows higher corrosion. 2018/2019 70 Metals and Dental Alloys
- 71. Stomatological solders Due to very high toxicity of cadmium the earlier very common CADMIUM solders are now prohibited. The most dangerous are the cadmium vapors, which are formed during soldering and may cause chronic intoxication in dental laboratory technicians. (cancerogenicity !!) 2018/2019 71 Metals and Dental Alloys


































































![Materials for dental implants
Ceramic
Ceramic materials are fully oxidized, and therefore
chemically very stable.
Solely ceramic implants may be used, or the ceramic
materials may be used for coating of metallic materials.
So called bioactive ceramic materials, which react with the
bone and merge together are introduced now. They contain
oxyapatite a fluoroapatite [Ca10(PO4)6)O,F2], -wollastonite
(SiO2-CaO) in MgO-CaO-SiO2 glass matrix.
Further advantage is low thermal and electrical conductivity
and similar elasticity as the bone.
In the literature they are usually labeled as CPC (calcium
phosphate ceramics).
2018/2019 68
Metals and Dental Alloys](https://image.slidesharecdn.com/15446183-230326092503-927ef5eb/85/15446183-ppt-68-320.jpg)


