31-P NMR SPECTROSCOPY
- 1. 1
- 2. 2 By MUHAMMAD AKRAM PhD Chemistry Worthy Teacher : Dr. UZMA YUNUS (Associate Professor) Allama Iqbal Open University Islamabad
- 3. Presentation Layout 3 Introduction to 31P NMR Spectroscopy Chemical Shifts , Coupling Constants Coupling other Nuclei Applications
- 4. 4 Phosporus-31 nuclear magnetic resonance is an analytical technique used to identify phosphorus-containing compounds, such as organic compounds and metal complexes. Phosphorus -31 nuclear magnetic resonance (31P NMR) conceptually same as (1H NMR). 31P is one of the most important nuclei for NMR spectroscopy due to its nuclear spin of 1/2, high gyromagnetic ratio ( 17.235 M Hz T-1 ) and has100% natural isotopic abundance. Introduction of 31P NMR Spectroscopy
- 5. 5 I Atomic Mass Atomic Number Nuclei Half-integer Odd Odd 1 1H(½), 31 15P(½) Half-integer Odd Even 13 6 C(½), 73 32Ge(9/2) Integer Even Odd 14 7 N(1), 2 1 H(1) Zero Even Even 12 6 C(0), 16 8 O(0), 32 16S(0) Table 1.1 A quick guide as to whether an isotope has zero, half-integer, or integer nuclear spin Nuclei with I = 1/2 are called dipolar nuclei. They appear to be spherical, with a uniform charge distribution over the entire surface. Since the nucleus appears to be spherical, it disturbs a probing electromagnetic field independent of direction. The result is a strong, sharp NMR signal. 31P is a dipolar nucleus. Nuclei with I > 1/2 are called quadrupolar nuclei. They have a non- spherical charge distribution, and rise to non-spherical electric and magnetic fields. They have a quadruple moment. Introduction of 31P NMR Spectroscopy
- 6. 6 Nucleus Natural Abundance [%] Magnetic Moment μ [μ N] Magnetogyric Ratio γ[107 rad T−1 s−1] NMR frequency MHz Standard 1H 99.985 4.83724 26.7519 100.000000 TMS 13C 1.108 1.2166 6.7283 25.145004 TMS 15N 0.37 −0.4903 −2.712 10.136783 MeNO2, [NO3 19F 100 4.5532 25.181 94.094003 CCl3F 9Si 4.70 −0.96174 −5.3188 19.867184 TMS 31P 100 1.9602 10.841 40.480737 85% H3PO4 From Table, it can be seen that the magnetogyric ratio for phosphorus is about 2.5 times smaller than that for hydrogen. The required radiofrequency is therefore also about 2.5 times smaller than that of the spectrometer. For a 400 MHz NMR spectrometer, that would calculate to approximately 161 MHz Introduction of 31P NMR Spectroscopy
- 7. 7 Normally chemical shifts in 31P NMR is typically reported relative to 85% phosphoric acid ( δ = 0 ppm) which is used as external standard due its reactivity. They are also sensitive to temperature (and pressure). Reference standard for 31P-NMR spectroscopy is 85% H3 PO4(external), meaning that a sealed ampule containing 85% aqueous H3 PO4 submerged in a NMR tube filled with D2O is measured and stored on the NMR spectrometer’s hard drive as a reference. Standard in 31P NMR Spectroscopy
- 8. 8 200 150 100 50 0 -50 -100 RPH2 R2PH R3P R2PX P(NR)3 P(OR)3 P(SR)3 PX3 P(OR)2X CPX2 CPXN O=PX3 O=P(OR)X2 O=P(OR)2X O=P(OR)3 S=P(OR)3 O=P(R)(OR) S=P(R))(OR) O=P(R)(OH) (RO)2POH 200 150 100 50 0 -50 -100 ( δ ppm) .Chemical shift range of different type of phosphorus compounds
- 9. 9 The range of 31P chemical shifts in diamagnetic compounds covers some 2000 ppm, and is thus one order of magnitude larger than that of carbon, and two orders of magnitude larger than proton. The upfield end in 31P –NMR is due to white phosphorus P4 at δP= −527 to −488 ppm, depending on solvent and water content of the sample. Chemical shift values of Phosphorus resonance also dependence upon the bond angle, electronegativity of the substituent's and the p-bonding character of the substituent's . From 13C-NMR, we know that an olefin resonates downfield from an alkane, and an acetylene is found in between, but closer to the alkane. This is explained by diamagnetic anisotropy and the behavior of P-C, P=C, and P≡C bonds also same. Chemical Shift Values in 31P NMR Spectroscopy
- 10. 10 Tertiary phosphanes (PMe3 , PEt3, PPrn 3 , PBun 3, and PBut 3 ) showing the continuous downfield shift as the length of the alkyl chain increases. The last phosphane in the series, PBut 3 , should show an additional contribution for steric effect. The actual chemical shift values are δP = −62 ppm, δP = −20 ppm, δP = −33 ppm, δP =−33 ppm, and δP =63ppm, meaning that from going to methyl to n-propyl, the chemical shift difference is Δδ = −13 ppm upfield, and not downfield as expected. Chemical shift difference between n-propyl and n-butyl (Δδ = 0 ppm), PBut 3,is Δδ =83ppm downfield from PEt3 , and thus in the expected region, if one takes the observed chemical shift difference between PMe3 and PEt3 (Δδ = 42 ppm downfield) as representative Dependence of 31P-NMR chemical shifts on the number of hydrogen substituent's
- 11. 11 At the upfield end, white phosphorus P4 resonates at δP = −488 ppm. This is readily explained by an electron rich cluster structure with P-P-P angles of 60°. Coordination to a transition metal causes a reduction in electron density on phosphorus, as a P4 lone pair is used to create a donor bond. Coordinating the phosphane PMe3 to a transition metal again results in the expected downfield shift of Δδ = 107 ppm, since the phosphorus lone pair is utilized for the donor bond, resulting in a decrease of electron density on phosphorus. Similarly, substitution of a phosphorus atom by an isolobal transition metal fragment causes a downfield shift, as electron density is transferred from the electron rich phosphorus atoms to the “poorer” transition metal. In general, negative coordination shifts are possible. The 31P chemical shift range for phosphorus
- 12. 12 However, when we form covalent bonds between phosphorus and the metal, the picture changes. Looking at [Cp2 Hf(PCy2 )2 ], we see two different phosphorus resonances at δP = 270.2 ppm and d P = −15.3 ppm, respectively. We note that the two phosphorus atoms are trigonalplanar and tetrahedrally coordinated, and suspect that they are part of a P = Hf double and a P-Hf single bond, respectively. The two 31P-NMR signals in [Cp2 Hf(PCy2 )2 ]
- 13. 13 31P-NMR chemical shift values for phosphorus carbon multiple bonds Normally five membered heteroaromatic ring systems ( i.e. pyrrole ) are electron rich, and the corresponding six membered (pyridine) ones are electron poor. The 1,3,5-triphosphabenzene resonates at δP = 232.6 ppm, considerably downfield from the benzazaphosphole at d P = 69.8 ppm, as expected. Interestingly, the 3,4 diphosphinidene-cyclobutene, featuring a conjugated, non-aromatic π-electron system with resonance structures, has a P chemical shift of δP = 147.0 ppm midway between the two. The analog to 1,3,5-triphosphabenzene is stable, and shows P resonances at δP = 93.6 and 336.8 ppm. These 31P chemical shifts are evidence for the folded roof-like structure of the compound, featuring a tertiary phosphane phosphorus atom at δP = 93.6 ppm, and an isolated P=C at δP = 336.8ppm, respectively. In 13C-NMR, chemical shift of benzene at δP = 128 ppm with that of the cyclopentadienide ligand Cp− at δP = 118–136 ppm, depending on the cationic metal that it binds to. In 31P-NMR, 1,3,5-triphosphabenzene with a P resonate at δP = 232.6 ppm, and 1,3,6 triphosphafulvenide anion, the relevant P atoms resonating at δP = 216, 223 ppm.
- 14. 14 The influence of coordination to a Lewis acid on the 31P chemical shift Two phosphanes for comparison, PMe3 and PPh3, and note that the downfield shift for PPh3 upon coordination ranges from Δδ = 21–40 ppm, and that for PMe3 is in an even narrower band of Δδ = 46–60 ppm. In each case, the actual downfield shift depends on the Lewis base. Factors are the geometry around the metal (cis or trans), and the substituent's on phosphorus (P+ or PPh2 + ).
- 15. 15 Phosphorus PCl3 PCl2F PClF2 PF3 Trihalides δ P [ppm] 220 224 176 97 Phosphorus PI3 PBr3 PCl3 PF3 P(CN)3 Trihalides δ P [ppm] 178 227 218 97 −136 O = PBr3 O = PCl3 O = PF3 δ P [ppm] −102.9 2 −35.5 The 31P chemical shift values for selected phosphorus trihalides The explanation is a π-bonding interaction (hyper conjugation) between the fluorine lone pair and phosphorus, resulting in an increase of electron density on phosphorus, and thus an upfield shift in the phosphorus resonance. Substituent's capable of π-bonding interactions cause additional shifts: upfield for a donor interaction, and downfield for an acceptor interaction. The influence of the halogen on the phosphorus resonance of the phosphorus trihalide
- 16. 16 Generally larger in magnitude than those in 1 H- or 13C-NMR spectroscopy, but they are governed by the same principles. 1 J coupling constants of 1000 Hz are readily observed. Coupling is facilitated through the s-bonds of the backbone. There is no pronounced π-effect as the one observed for the chemical shift values, but there are examples for which a limited π-effect is indeed observed. As with the more common nuclei, 1 H and 13C, the phosphorus coupling constants decrease with an increase of bonds between the coupling nuclei. However, it is frequently observed that 3 JPX coupling constants are larger than 2 JPX coupling constants. In the following we will examine the trends in the coupling constants of phosphorus with the more common nuclei 1 H, 13C, and 31P. The coupling constants in 31P-NMR spectroscopy
- 17. 17 As with other nuclei, n JPH coupling constants have a tendency to decrease when the value of n increases. Although 1 JPH values are frequently in the range of 400–1000 Hz, the coupling constants drop rapidly and are detectable for 4 JPH only in special circumstances. JPH Coupling Constants value for 1 JPH increases with decreasing electron density on phosphorus. In the series PH4 + , PH3 , and PH2 − , the 1 JPH coupling constants are 547 Hz, 189 Hz, and 139 Hz, respectively. It appears that the influence of electron lone pairs on the 1 JPH coupling constant becomes smaller with each lone pair. Δ 1 JPH for PH4 + /PH3 is 358 Hz, whereas for PH3 /PH2 − Δ, 1 JPH is only 50 Hz. When the phosphorus binds to a σ-acceptor, such as a transition metal or a main group Lewis acid. As a result, the electron density on P drops sharply and the 1 JPH coupling constant increases accordingly. A good example is with PH3 , which has a 1 JPH =189 Hz as a free ligand, but a significantly larger one in its complexes: 307 Hz in [Cr(CO)4 (PBu3 )(PH3 )], and 366 Hz in Me3 BPH3 , respectively. Note: PF2 H (1 JPH = 181.7 Hz) has a smaller 1 JPH coupling constant than PH3 (1 JPH = 189 Hz) despite the greater electronegativity of fluorine compared to hydrogen. The reason, of course is π-back-bonding (hyperconjugation) observed in P-F, but not in P-H bonds, decreasing the effective electronegativity of F towards P . Similarily, the 1 JPH coupling constant increases with an increasing sum of the electronegativities of the substituent's on phosphorus.: H < alkyl < aryl < OR < OPh. Note: The 1 JPH coupling constant increases with decreasing steric bulk of the substituent's on phosphorus Phosphorus coupling to Hydrogen
- 18. 18 In 2 JPH (H-A1-P) and 3 JPH ( H-A1-A1-P) commonly feature is a minimum of the coupling constant around Θ = 90° with maxima at Θ = 0° and Θ = 180°, respectively. Typically, the maximum at Θ = 180° is larger than at Θ = 0°. The relationship between the magnitudes of 2 JPH (gem) and 3 JPH (cis and trans) coupling constants is similar to that observed in JHH coupling constants. 3 JPH (trans) is frequently larger than 3 JPH (cis), but 2 JPH (gem) can be larger than 3 JPH (trans). The electron density on phosphorus has a pronounced effect on 2 JPH (gem) and 3 JPH (cis). Coordination to a transition metal markedly increases both, whereas electronegative substituent's on phosphorus effect only 3 JPH (cis) in a variable manner. The effect on 3 JPH (trans) is small, but nonetheless significant. The coupling constants for 3 JPH (trans) are typically 10–30 Hz, 2 JPH (gem) up to 25 Hz, and 3 JPH (cis) up to 15 Hz. Without electronegative substituent's on phosphorus, the values for 2 JPH (gem) and 3 JPH (cis) remain well under 10 Hz. Phosphorus coupling to Hydrogen
- 19. 19 2,6,7-trioxa-1,4- dicyclo[2.2.2.]Octane Phosphorus shifts at 90.0 ppm for Pά and -67.0 ppm for Pβ Phosphorus coupling to Hydrogen
- 20. 20 Phosphorus coupling to Hydrogen
- 21. 21 (PhCH2 ) 2 PPh PhCH2 (Ph)2 P Ph3 P Ph4 P+ Ph3 P = O 1 JPC [Hz] 20.6 16.2 −12.5 88.4 104.4 2JPC [Hz] 19.8 18.9 19.6 10.9 9.8 3 JPC [Hz] 7.0 6.4 6.8 12.8 12.1 4JPC [Hz] 0.6 0.2 0.3 2.9 2.8 Coupling to Carbon The coupling of phosphorus to carbon follows the general trends observed with other nuclei. The magnitude of n J PC is dependent on the oxidation state and the coordination number of phosphorus .The electronegativity of substituent's on phosphorus also plays an important role. The magnitude of 1JPC increases dramatically upon loss of the lone pair on phosphorus. 3JPC becomes larger than 2 JPC upon loss of the lone pair on phosphorus. For methyl phosphorus derivatives, the 1JPC coupling constant for compounds with phosphorus in the oxidation state +III is generally found to be small or even negative, whereas the corresponding 1 JPC coupling constant for phosphorus compounds where the phosphorus is in the oxidation state +V is usually substantially larger and positive.
- 22. 22 Coordinated 1JPC 2 JPC 3 JPC 2 JPC 1JPC 3 JPC n JPC Free ligand Influence of coordination on n JPC Upon coordination to transition metals, the nJPC coupling constants in trialkyl phosphanes are influenced differently. The 1JPC coupling constant increases substantially, the 2JPC coupling constant decreases substantially, and the 3JPC coupling constant increases slightly. A typical example is shown in Fig. Coupling to Carbon
- 23. 23 Coupling to Hydrogen and Carbon
- 24. 24 Coupling to Phosphorus JPP in organ phosphorus compounds cover a wide range of values , where as P2 Me4 1JPP = −180 Hz, this value drops to 1 JPP = −290 Hz for ButMePPMeBut , 1 JPP = −318 Hz for Me2 PPBut 2 , and as low as 1 JPP = −451 Hz for P2But4 . The corresponding doubly oxidized derivatives R2 (E)PP(E)R2 (E = O, S, Se) follow the same trend, but they can be found to the left (larger coupling constants) from the non-oxidized analogues. P2 Me4 S2 has a coupling constant of 1 JPP = −19 Hz, and {P(S) MeBut}2 a coupling constant of 1 JPP = −109 Hz. • Finally, the monoxidized diphosphanes R2 (E)PPR2 (E = O, S, Se) have similar 1 JPP coupling constants to the diphosphanes, indicating that one PALP is sufficient for the magnitude of the 1 JPP coupling constant. Note: Steric crowding results in a decrease of the 1 JPP coupling constant in diphosphanes. Note: Lower electron density results in an increase of 1 JPP constants in diphosphanes.
- 25. 25 The energy supply in cells is provided by molecules that contain phosphorus. Especially PCr (Phosphocreatine) and ATP (Adenosine-Tri-Phosphate) carry the energy needed for most processes in the brain and other organs. Magnetic Resonance Spectroscopy has the potential to quantitatively detect these substances in living subjects noninvasively. Spectra of some Phosphorus compounds
- 26. 26 Spectra of some Phosphorus compounds
- 27. 27 Pt Coupling to Phosphorus
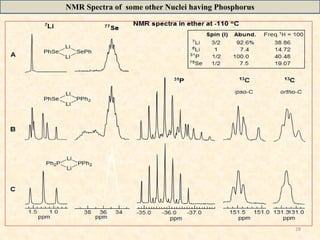
- 28. 28 NMR Spectra of some other Nuclei having Phosphorus
- 29. 29 Applications: Monitoring the Reaction Single signal at -4.40 ppm corresponding to free diphosphine ligand. After an hour a new signal appear at 40.05 ppm , corresponding to diphosphine nickel complex. The downfield peak grows as the reaction proceeds relative to the up field peak. No change is observed between four and five hours, suggesting the conclusion of the reaction. -4.40 ppm 40.05 ppm Coskran J.K.; Verkade G.j. Inorganic. Chem. 1965, 4,1665.
- 30. 30 Applications: Determine the Epoxide content of carbon nonmaterial's An Epoxide react with methyltrioxorhenium to form a five membered ring. In the presence of triphenyl phosphine (PPh3) , the catalyst is regenerated, forming an alkene and triphenyl phosphine oxide (OPPh3) the same reaction can be applied to carbon nanostructures and used to quantify the amount of Epoxide on the nonmaterial's. 31 P spectrum of experiment before addition of Re complex (top) and at the completion of experiment (bottom). The integration of the two 31P signals is used to quantify the amount of Epoxide on the nanotube according to equation. Verkade, G.J.; Quin D.L. Phosphorus -31-NMR Spectroscopy in seterochemical analysis. 1987.
- 31. 31 Applications 31P NMR spectra of the reaction products catalysed by PhnI, PhnM and PhnJ. a, RPnTP from the reaction of methylphosphonate and MgATP catalysed by PhnI in the presence of PhnG, PhnH, and PhnL at pH 8.5. The methylphosphonyl group is labelled as 1-Pn. Inset, 31P–31P coupling of the triphosphate portion of the RPnTP product (the ά, β and γ phosphoryl groups). b, The formation of PRPn from RPnTP in the presence of PhnM. Inset, protoncoupled spectrum at pH 8.5 showing the multiplet that corresponds to 1-Pn and the triplet that corresponds to the 5-phosphate (5P). Pi , inorganic phosphate. c, The formation of PRcP from PRPn in the presence of PhnJ at pH 6.8. Inset, proton-coupled spectrum showing the formation of a new triplet that corresponds to the 1,2-cyclic moiety of PRcP (1,2- cP). The chemical shifts for the phosphate moiety at the fifth carbon atom of PRcP and PRPn are coincident with one another (3.4 ppm). Reaction pathway for the conversion of methylphosphonate to PRcP. The proteins PhnG, PhnH, PhnI, PhnJ, PhnL and PhnM are required for this transformation. The role of PhnK is unknown. PhnGHL denotes PhnG, PhnH and PhnL. Siddhesh S. K.; , Howard J.; Frank M. R.; Intermediates in the transformation of phosphonates to phosphate by bacteria. Nature. 2011 , 480, 570- 573.
- 32. 32 1D 31P NMR spectra of NaOH/EDTA extracts of soil-1, which is naturally high in Fe. Spectra without (bold line) and with (thin line) sulfide treatment are compared. The region displayed corresponds to phosphomonoesters and inorganic phosphate (6.5 ppm). Spectra are scaled for easy comparison. Applications Organic phosphorus (P) compounds represent a major component of soil P in many soils and are key sources of P for microbes and plants. Solution NMR (nuclear magnetic resonance spectroscopy) is a powerful technique for characterizing organic P species. Overlap is often exacerbated by the presence of paramagnetic metal ions, even if they are in complexes with EDTA following NaOH/EDTA extraction Johan V.; Andrea G. V. High-Resolution Characterization of Organic Phosphorus in Soil Extracts Using 2D 1 H−31P NMR Correlation Spectroscopy. Environ. Sci. Technol. 2012, 46, 3950−3956
- 33. 33 Applications
- 34. 34 Possible binding modes for 1·Zn2222 with PPi and ATP suggested by31P NMR data. Fig. 31P NMR spectra (202 MHz) of ATP (10 mM) in the absence and presence of receptor 1·Zn2222 in D2O at 300 K. (a) [1·Zn2222] = 0. (b) [1·Zn2222] = 0.5 equiv. (c) [1·Zn2222] = 1 equiv. (d) [1·Zn2222] = 2 equiv. Applications
- 35. 35 Enlarged portions of in vivo proton-decoupled 31P-NMR spectra of sycamore cells. (A) Cells harvested after a 2-wk preincubation in Mg2+ -free NM and perfused with Mg2+-free NM. Acquisition time, 4 h (24,000 scans). The spectrum is the sum of four successive comparable 1-h spectra. Peak 1 was attributed to the γ-signal of mitochondrial ATP, and the broad peak 2 was attributed to the β-signal of ADP pools present in mitochondria and cytosol. (B) Spectrum accumulated 1 h after the addition of 1 mM MgSO4 to NM. Perfusion conditions are as with standard NM. Acquisition time, 1 h (6,000 scans). Horizontal arrows indicate the downfield shift (toward the left of the spectra) of the different ATP resonance peaks after the addition of MgSO4 to NM. (Insets) Centered on γ-ATP at −6.2 ppm, portions of PCA extract spectra prepared from cells incubated outside the magnet under the same conditions. Acquisition time, 1 h (1,024 scans) Elisabeth G.; Fabrice R.; Roland D.; Richard B. Interplay of Mg2+, ADP, and ATP in the cytosol and mitochondria: Unravelling the role of Mg2+ in cell respiration 19, 2014. Applications
- 36. 36 Applications
- 37. 37 Applications A normal spectra of a forearm human muscle displaying 6 pics and their variation during a rest - exercise -recovery protocol. The major peaks of the Phosphorus-MR spectrum are: phosphocreatine (PCr), inorganic phosphates (Pi), phosphodiesters (PDE), and the three peaks (α, β, γ) of ATP. The chemical shift (in parts per million [ppm]) of the Pi is used for calculation of intracellular, cytosolic pH. Note the decrease of PCr concentration and the increase of Pi concentration during exercise. Mattei J.P.; Bendahan D .; Cozzone P. P-31 Magnetic Resonance Spectroscopy. A tool for diagnostic purposes and pathophysiological insights in muscle diseases. Reumatismo, 2004; 56(1):9-14 Mitochondrial Disorders. Genetic and biochemical heterogeneity. A majority of these diseases include neurological symptoms besides. Glycolytic Defects. painful cramps, contractures, and with progressive weakness.
- 38. 38 A) Fully relaxed 31P spectrum without ST preparation (tsat = 0 s) (left) and 31P- ST Spectrum with complete saturation (arrow) of the γ-ATP resonance (tsat = 6.84 s) (right). B) Series of ST spectra at different tsat. C) PCr signal intensity fitted to Eq.2, (Pearson's product moment correlation = 0.9992). In order to estimate the pseudo first-order forward rate constant (kf) of the CK reaction, we need to measure the phosphocreatine (PCr) signal while we saturate the γ-adenosine triphosphate (γ-ATP) resonance for different durations (i.e. the progressive ST experiment) Assuming a two-pool exchange system between PCr and γ-ATP and complete saturation of γ- ATP, we can estimate the exchange rate between the two metabolites by solving the modified, for chemical exchange, Bloch equation . To confirm the efficiency of our saturation pulses, we acquired unlocalized 31P spectra in the entire volume of the lower leg muscles in all of our volunteers. Prodromos P. ; Ding X.; Gregory C.; Ravinder R. Three-dimensional Saturation Transfer31P-MRI in Muscles of the Lower Leg at 3.0 T Scientific Reports. 2014 ApplicationsApplications
- 39. 39 Static 31P spectra of DMPC (a–c) and VM (d–i) membranes in the absence and presence of M2 at 303 K. (a, d, and g) Protein-free lipid bilayers. (b, e, and h) M2TM-containing membranes. (c, f, and i) M2(21–61)-containing membranes. The DMPC spectra (a–c) and the VM spectra (d–f) were measured on samples with Amt, whereas the VM spectra (g, h, and i) were measured on samples without Amt. Applications NMR Determination of Protein Partitioning into Membrane Domains with Different Curvatures and Application to the Influenza M2 Peptide. Many membrane peptides and proteins cause curvature to the phospholipid bilayer as their mechanism of action. Examples include viral fusion proteins The M2 protein of the influenza A. Transmembrane (TM) 1,2-dimyristoyl-sn-glycero-3-phosphocholine bilayers. (DMPC) Tuo W.; Sarah D. C.; Mei H.NMR Determination of Protein Partitioning into Membrane Domains with Different Curvatures and Application to the Influenza M2 Peptide. Biophysical Journal. 2012, 102(4) ,787–794
- 40. 40 Applications Single-voxel 1 H MRS and 31P MRSI overlaid on MRI acquired from the second patient during neoadjuvant chemotherapy. Not only can the conventionally obtained modulation in total choline (tCho) (spectra, left) and tumor volume (indicated by the dotted area) be observed, but also alterations in the levels of phosphoethanolamine (PE), glycerol phosphocholine (GPC), glycerol phosphoethanolamine (GPE) and inorganic phosphate (Pi) (spectra, right). Prior to treatment, 31P resonances of PE, GPC, GPE and Pi were restricted to the tumor area, whereas, after the first dose of chemotherapy, mainly levels of Pi remained detectable. As can be seen, these Pi levels were not restricted to the tumor region, but were similarly elevated in glandular tissue. At the end of the last dose of chemotherapy, but prior to surgery, the recurrence of phospholipid levels was detected in the tumor. Dennis W. J. K.; Bart B.D. 31P MRSI and 1 H MRS at 7 T: initial results in human breast cancer. NMR Biomed. 2011; 24: 1337–1342.
- 41. 41






![6
Nucleus Natural
Abundance
[%]
Magnetic Moment
μ [μ N]
Magnetogyric Ratio
γ[107 rad T−1 s−1]
NMR frequency
MHz
Standard
1H 99.985 4.83724 26.7519 100.000000 TMS
13C 1.108 1.2166 6.7283 25.145004 TMS
15N 0.37 −0.4903 −2.712 10.136783 MeNO2,
[NO3
19F 100 4.5532 25.181 94.094003 CCl3F
9Si 4.70 −0.96174 −5.3188 19.867184 TMS
31P 100 1.9602 10.841 40.480737 85%
H3PO4
From Table, it can be seen that the magnetogyric ratio for phosphorus is
about 2.5 times smaller than that for hydrogen.
The required radiofrequency is therefore also about 2.5 times smaller than that of
the spectrometer. For a 400 MHz NMR spectrometer, that would calculate to
approximately 161 MHz
Introduction of 31P NMR Spectroscopy](https://image.slidesharecdn.com/akramppt-160312122959/85/31-P-NMR-SPECTROSCOPY-6-320.jpg)





![12
However, when we form covalent bonds between phosphorus and the metal, the picture
changes. Looking at [Cp2 Hf(PCy2 )2 ], we see two different phosphorus resonances at
δP = 270.2 ppm and d P = −15.3 ppm, respectively.
We note that the two phosphorus atoms are trigonalplanar and tetrahedrally coordinated,
and suspect that they are part of a P = Hf double and a P-Hf single bond, respectively.
The two 31P-NMR signals in [Cp2 Hf(PCy2 )2 ]](https://image.slidesharecdn.com/akramppt-160312122959/85/31-P-NMR-SPECTROSCOPY-12-320.jpg)


![15
Phosphorus PCl3 PCl2F PClF2 PF3
Trihalides
δ P [ppm] 220 224 176 97
Phosphorus PI3 PBr3 PCl3 PF3 P(CN)3
Trihalides
δ P [ppm] 178 227 218 97 −136
O = PBr3 O = PCl3 O = PF3
δ P [ppm] −102.9 2 −35.5
The 31P chemical shift values for selected phosphorus trihalides
The explanation is a π-bonding interaction
(hyper conjugation) between the fluorine lone
pair and phosphorus, resulting in an increase of
electron density on phosphorus, and thus an
upfield shift in the phosphorus resonance.
Substituent's capable of π-bonding interactions
cause additional shifts: upfield for a donor
interaction, and downfield for an acceptor
interaction.
The influence of the halogen on the phosphorus resonance of the phosphorus trihalide](https://image.slidesharecdn.com/akramppt-160312122959/85/31-P-NMR-SPECTROSCOPY-15-320.jpg)

![17
As with other nuclei, n JPH coupling constants have a tendency to decrease when the value of n increases.
Although 1 JPH values are frequently in the range of 400–1000 Hz, the coupling constants drop rapidly
and are detectable for 4 JPH only in special circumstances.
JPH Coupling Constants value for 1 JPH increases with decreasing electron density on phosphorus. In the
series PH4 + , PH3 , and PH2 − , the 1 JPH coupling constants are 547 Hz, 189 Hz, and 139 Hz, respectively.
It appears that the influence of electron lone pairs on the 1 JPH coupling constant becomes smaller with
each lone pair. Δ 1 JPH for PH4 + /PH3 is 358 Hz, whereas for PH3 /PH2 − Δ, 1 JPH is only 50 Hz.
When the phosphorus binds to a σ-acceptor, such as a transition metal or a main group Lewis acid. As a
result, the electron density on P drops sharply and the 1 JPH coupling constant increases accordingly.
A good example is with PH3 , which has a 1 JPH =189 Hz as a free ligand, but a significantly larger one in
its complexes: 307 Hz in [Cr(CO)4 (PBu3 )(PH3 )], and 366 Hz in Me3 BPH3 , respectively.
Note: PF2 H (1 JPH = 181.7 Hz) has a smaller 1 JPH coupling constant than PH3 (1 JPH = 189 Hz) despite the
greater electronegativity of fluorine compared to hydrogen. The reason, of course is π-back-bonding
(hyperconjugation) observed in P-F, but not in P-H bonds, decreasing the effective electronegativity of F
towards P .
Similarily, the 1 JPH coupling constant increases with an increasing sum of the electronegativities of the
substituent's on phosphorus.: H < alkyl < aryl < OR < OPh. Note: The 1 JPH coupling constant increases
with decreasing steric bulk of the substituent's on phosphorus
Phosphorus coupling to Hydrogen](https://image.slidesharecdn.com/akramppt-160312122959/85/31-P-NMR-SPECTROSCOPY-17-320.jpg)

![19
2,6,7-trioxa-1,4-
dicyclo[2.2.2.]Octane
Phosphorus shifts at 90.0 ppm for Pά
and -67.0 ppm for Pβ
Phosphorus coupling to Hydrogen](https://image.slidesharecdn.com/akramppt-160312122959/85/31-P-NMR-SPECTROSCOPY-19-320.jpg)

![21
(PhCH2 ) 2 PPh PhCH2 (Ph)2 P Ph3 P Ph4 P+ Ph3 P = O
1 JPC [Hz] 20.6 16.2 −12.5 88.4 104.4
2JPC [Hz] 19.8 18.9 19.6 10.9 9.8
3 JPC [Hz] 7.0 6.4 6.8 12.8 12.1
4JPC [Hz] 0.6 0.2 0.3 2.9 2.8
Coupling to Carbon
The coupling of phosphorus to carbon follows the general trends observed with other
nuclei. The magnitude of n J PC is dependent on the oxidation state and the
coordination number of phosphorus .The electronegativity of substituent's on
phosphorus also plays an important role.
The magnitude of 1JPC increases dramatically upon loss of the lone pair on phosphorus.
3JPC becomes larger than 2 JPC upon loss of the lone pair on phosphorus. For methyl
phosphorus derivatives, the 1JPC coupling constant for compounds with phosphorus in the
oxidation state +III is generally found to be small or even negative, whereas the
corresponding 1 JPC coupling constant for phosphorus compounds where the phosphorus is in
the oxidation state +V is usually substantially larger and positive.](https://image.slidesharecdn.com/akramppt-160312122959/85/31-P-NMR-SPECTROSCOPY-21-320.jpg)











![34
Possible binding modes for 1·Zn2222 with PPi and ATP suggested by31P NMR data.
Fig. 31P NMR spectra (202 MHz) of ATP (10 mM) in the absence and presence of receptor 1·Zn2222 in D2O at 300
K. (a) [1·Zn2222] = 0. (b) [1·Zn2222] = 0.5 equiv. (c) [1·Zn2222] = 1 equiv. (d) [1·Zn2222] = 2 equiv.
Applications](https://image.slidesharecdn.com/akramppt-160312122959/85/31-P-NMR-SPECTROSCOPY-34-320.jpg)


![37
Applications
A normal spectra of a forearm human muscle
displaying 6 pics and their variation during a rest -
exercise -recovery protocol. The major peaks of the
Phosphorus-MR spectrum are: phosphocreatine
(PCr), inorganic phosphates (Pi), phosphodiesters
(PDE), and the three peaks (α, β, γ) of ATP. The
chemical shift (in parts per million [ppm]) of the Pi
is used for calculation of intracellular, cytosolic pH.
Note the decrease of PCr concentration and the
increase of Pi concentration during exercise.
Mattei J.P.; Bendahan D .; Cozzone P. P-31 Magnetic Resonance Spectroscopy. A tool for diagnostic purposes and pathophysiological insights in muscle diseases. Reumatismo, 2004; 56(1):9-14
Mitochondrial Disorders.
Genetic and biochemical
heterogeneity. A majority of
these diseases include
neurological symptoms
besides.
Glycolytic Defects. painful
cramps, contractures, and with
progressive weakness.](https://image.slidesharecdn.com/akramppt-160312122959/85/31-P-NMR-SPECTROSCOPY-37-320.jpg)



