4910461.ppt
- 1. Chapter 20 Transition Elements and Coordination Chemistry
- 2. Chapter 20 Slide 2 Chem 1A Review Why Study Coordinated Complexes of Transition metals? These compounds are used as catalyst in oxidation of organic compounds and pharmaceutical applications.
- 3. Chapter 20 Slide 3 Order of orbitals (filling) in multi-electron atom 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f< 5d< 6p< 7s< 5f< 6d< 7p
- 4. Chapter 20 Slide 4
- 5. Chapter 20 Slide 5 ns 1 ns 2 ns 2 np 1 ns 2 np 2 ns 2 np 3 ns 2 np 4 ns 2 np 5 ns 2 np 6 d 1 d 5 d 10 4f 5f Electron Configuration and the Periodic Table
- 6. Slide 6 a)S [Ne]3s23p4 Using periodic table write Noble gas notation for the following elements: b)Fe [Ar] 4s23d6 c)Se [Ar] 4s23d104p4 d)Gd [Xe]6s24f75d1 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f< 5d< 6p< 7s< 5f< 6d< 7p
- 7. Chapter 20 Slide 7
- 8. Chapter 20 Slide 8 Electron Configurations
- 9. Chapter 20 Slide 9 Why Study Coordinated Complexes of Transition metals? Are used as catalyst in oxidation of organic compounds. Medicinal applications. “Cisplatin” - a cancer chemotherapy agent
- 10. Chapter 20 Slide 10 Coordination Compounds Co(H2O)6 2+ Pt(NH3)2Cl2 Cu(NH3)4 2+ “Cisplatin” - a cancer chemotherapy agent
- 11. Chapter 20 Slide 11 Coordination Compounds of Ni2+ [Ni(NH2C2H4NH2)3]+2 [Ni(NH3)6]+2
- 12. Chapter 20 Slide 12 Electron Configurations Zn (Z = 30): [Ar] 3d10 4s2 Sc (Z = 21): [Ar] 3d1 4s2
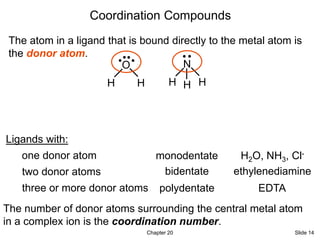
- 13. Chapter 20 Slide 13 Coordination Compounds Many coordination compound consists of a complex ion. A complex ion contains a central metal cation bonded to one or more molecules or ions. The molecules or ions that surround the metal in a complex ion are called ligands. A ligand has at least one unshared pair of valence electrons H O H •• H N H H •• • • Cl •• • • - • • C O • • Omit 20.2 - 20.4
- 14. Chapter 20 Slide 14 Coordination Compounds The atom in a ligand that is bound directly to the metal atom is the donor atom. H O H • • H N H H Ligands with: one donor atom monodentate two donor atoms bidentate three or more donor atoms polydentate H2O, NH3, Cl- ethylenediamine EDTA The number of donor atoms surrounding the central metal atom in a complex ion is the coordination number.
- 15. Chapter 20 Slide 15 Coordination Compounds Coordination Number: The number of ligand donor atoms that surround a central metal ion or atom.
- 16. Chapter 20 Slide 16 Ligands 02
- 17. Chapter 20 Slide 17 Ligands 03
- 18. Chapter 20 Slide 18 Poly DentateLigands • EDTA4– is often used to treat heavy metal poisoning such as Hg2+, Pb2+, and Cd2+. • EDTA4– bonds to Pb2+, which is excreted by the kidneys as [Pb(EDTA)]2–.
- 19. Chapter 20 Slide 19 Coordination Compounds H2N CH2 CH2 NH2 •• •• bidentate ligand polydentate ligand (EDTA) Bidentate and polydentate ligands are called chelating agents
- 20. Chapter 20 Slide 20 What is the Oxidation Numbers of Cu? Knowing the charge on a complex ion and the charge on each ligand, one can determine the oxidation number for the metal. Naming coordinated complex compounds +2
- 21. Chapter 20 Slide 21 Rule Applies to Statement 1 Elements The oxidation number of an atom in an element is zero. 2 Monatomic ions The oxidation number of an atom in a monatomic ion equals the charge of the ion. 3 Oxygen The oxidation number of oxygen is –2 in most of its compounds. (An exception is O in H2O2 and other peroxides, where the oxidation number is – 1.) Oxidation Number Rules
- 22. Chapter 20 Slide 22 Oxidation Number Rules Rule Applies to Statement 4 Hydrogen +1, it will be -1 when hydrogen comes with metal. NaH 5 Halogens Fluorine is –1 in all its compounds. Each of the other halogens is –1 in binary compounds unless the other element is oxygen. 6 Compounds and ions The sum of the oxidation numbers of the atoms in a compound is zero. The sum in a polyatomic ion equals the charge on the ion.
- 23. Chapter 20 Slide 23 What is the charge on the following Complex, If the Oxidation number of Cr is +3? Or, knowing the oxidation number on the metal and the charges on the ligands, one can calculate the charge on the complex ion.
- 24. Chapter 20 Slide 24 What are the oxidation numbers of the metals in K[Au(OH)4] and [Cr(NH3)6](NO3)3 ? OH- has charge of -1 K+ has charge of +1 ? Au + 1 + 4x(-1) = 0 Au = +3 NO3 - has charge of -1 NH3 has no charge ? Cr + 6x(0) + 3x(-1) = 0 Cr = +3
- 25. Chapter 20 Slide 25 Oxidation States of the 1st Row Transition Metals (most stable oxidation numbers are shown in red)
- 26. Chapter 20 Slide 26 Learning Check A complex ion contains a Cr3+ bound to four H2O molecules and two Cl– ions. Write its formula. +1
- 27. Chapter 20 Slide 27 Coordination Sphere • Coordinate bond: • Coordination Sphere: is the central metal and surrounding ligands. The square brackets separate the complex from counter ions such as SO4 2–. H H H H H H H H + H H H H Ag+ (aq) 2 Ag + [Ag(NH3)2]2 SO4 N N N
- 28. Chapter 20 Slide 28 Geometry of Coordination Compounds Coordination number Structure 2 4 6 Linear Tetrahedral or Square planar (mostly d8 ) Octahedral
- 29. Chapter 20 Slide 29 Coordination Number of 7&8 • Geometry Pentagonal bipyramid Hexagonal bipyramid Coordination Number of 8 Coordination Number of 7
- 30. Chapter 20 Slide 30 Coordination Compounds • Geometries:
- 31. Chapter 20 Slide 31 Nomenclature Co(H2O)6 2+ Pt(NH3)2Cl2 Cu(NH3)4 2+ Hexaaquacobalt(II) Tetraamminecopper(II) diamminedichloroplatinum(II) H2O as a ligand is aqua NH3 as a ligand is ammine Systematic naming specifies the type and number of ligands, the metal, and its oxidation state.
- 32. Chapter 20 Slide 32 Ligand’s Names 01
- 33. Chapter 20 Slide 33
- 34. Chapter 20 Slide 34 Nomenclature
- 35. Chapter 20 Slide 35 Nomenclature • Systematic naming follows IUPAC rules: • If compound is a salt, name cation first and then the anion, just as in naming simple salts. • In naming a complex ion or neutral complex, name ligands first and then the metal. • If the complex contains more than one ligand of a particular type, indicate the number with the appropriate Greek prefix: di–, tri–, tetra–, penta–, hexa–.
- 36. Chapter 20 Slide 36 Nomenclature • If the name of a ligand itself contains a Greek prefix, (ethylenediamine or triphenylphosphine) put the ligand name in parentheses and use: bis (2), tris (3), or tetrakis (4). • Use a Roman numeral in parentheses, immediately following the name of the metal, to indicate the metal’s oxidation state. • In naming the metal, use the ending –ate if metal is in an anionic complex.
- 37. Chapter 20 Slide 37 Name the following Complexes: Pt( Tris(ethylenediamine)nickel(II) IrCl(CO)(PPh3)2 Carbonylchlorobis(triphenylphosphine)iridium(I) [Ni(NH2C2H4NH2)3]2+
- 38. Chapter 20 Slide 38 What is the systematic name of [Cr(H2O)4Cl2]Cl ? tetraaquadichlorochromium(III) chloride Write the formula of tris(ethylenediamine)cobalt(II) sulfate [Co(en)3]SO4
- 39. Chapter 20 Slide 39 1. Constitutional Isomers: Have different bonds among their constituent atoms. • Ionization Isomers : [Co(NH3)5Br]SO4 (violet compound with Co–Br bond), [Co(NH3)5 SO4]Br (red compound with Co–SO4 bond). • Linkage Isomers form when a ligand can bond through two different donor atoms. Consider [Co(NH3)5NO2]2+ which is yellow with the Co–NO2 bond and red with the Co–ONO bond. Constitutional Isomerism
- 40. Chapter 20 Slide 40 Linkage Isomerism Co H3N H3N NO2 NH3 NH3 NH3 2+ Co H3N H3N ONO NH3 NH3 NH3 2+ sunlight Such a transformation could be used as an energy storage device.
- 41. Slide 41 2.Stereoisomers • Geometric Isomers of Pt(NH3)2Cl2: In the cis isomer, atoms are on the same side. In the trans isomer, atoms are on opposite sides. DNA-damaging antitumor agents Inactive
- 42. Chapter 20 Slide 42 2.Stereoisomers Geometric Isomers have the same connections among atoms but different spatial orientations of the metal–ligand bonds. a)cis isomers have identical ligands in adjacent corners of a square. b)trans isomers have identical ligands across the corners from each other.
- 43. Chapter 20 Slide 43 • Geometric Isomers of [Co(NH3)4Cl2]Cl: Isomers
- 44. Chapter 20 Slide 44 Enantiomers
- 45. Chapter 20 Slide 45 • An object or compound is achiral if it has a symmetry plane cutting through the middle. Enantiomers •Enantiomers are stereoisomers of molecules or ions that are nonidentical mirror images of each other. •Objects that have “handedness” are said to be chiral, and objects that lack “handedness” are said to be achiral.
- 46. Chapter 20 Slide 46 Enantiomers
- 47. Chapter 20 Slide 47 Unpolarized light. © 2003 John Wiley and Sons Publishers
- 48. Chapter 20 Slide 48 Plane-polarized light. © 2003 John Wiley and Sons Publishers plane-polarized light
- 49. Chapter 20 Slide 49 Reflected glare is plane-polarized light. © 2003 John Wiley and Sons Publishers
- 50. Chapter 20 Slide 50 Polarizing sunglasses versus glare. © 2003 John Wiley and Sons Publishers
- 51. Chapter 20 Slide 51 The effect of polarizing lenses on unpolarized light. © 2003 John Wiley and Sons Publishers Courtesy Andy Washnik
- 52. Chapter 20 Slide 52 plane-polarized light
- 53. Chapter 20 Slide 53 Enantiomers and Molecular Handedness
- 54. Chapter 20 Slide 54 Enantiomers Enantiomers have identical properties except for their reaction with other chiral substances and their effect on plane-polarized light. •Enantiomers are often called optical isomers; their effect on plane-polarized light can be measured with a polarimeter.
- 55. Chapter 20 Slide 55 Enantiomers • Plane-polarized light is obtained by passing ordinary light through a polarizing filter. • In a polarimeter the plane-polarized light is passed through a chiral solution and the polarization plane measured with an analyzing filter. • If the plane rotates to the right it is dextrorotatory. • If the plane rotates to the left it is levorotatory. • Equal amounts of each are racemic.
- 56. Chapter 20 Slide 56 Isomers 01
- 57. Slide 57 Co H3N H3N NO2 NH3 NH3 NH3 2+ Co H3N H3N ONO NH3 NH3 NH3 2+ sunlight [Co(NH3)5Br]SO4 (violet), [Co(NH3)5 SO4]Br (red ). See next slide for Diastereoisomers
- 58. Chapter 20 Slide 58 Diasteromers
- 59. Chapter 20 Slide 59 Bonding in Complexes 01 • Bonding Theories attempt to account for the color and magnetic properties of transition metal complexes. Ni2+ Cu2+ Zn2+ Co2+ •Solutions of [Ni(H2O)6]2+, [Ni(NH3)6]2+, & [Ni(en)3]2+
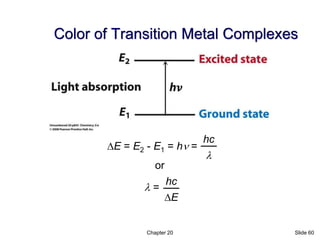
- 60. Chapter 20 Slide 60 Color of Transition Metal Complexes or DE hc l = DE = E2 - E1 = hn = l hc
- 61. Chapter 20 Slide 61 Color of Transition Metal Complexes
- 62. Chapter 20 Slide 62 Color of Transition Metal Complexes
- 63. Chapter 20 Slide 63 Bonding in Complexes: Valence Bond Theory
- 64. Chapter 20 Slide 64 Bonding in Complexes: Which empty orbital is metal using for bonding S, p, d or f ?
- 65. Chapter 20 Slide 65 Hybridization and sp3 Hybrid Orbitals How can the bonding in CH4 be explained? 4 valence electrons 2 unpaired electrons
- 66. Chapter 20 Slide 66 Hybridization and sp3 Hybrid Orbitals 4 valence electrons 4 unpaired electrons How can the bonding in CH4 be explained?
- 67. Chapter 20 Slide 67 Hybridization and sp3 Hybrid Orbitals 4 nonequivalent orbitals How can the bonding in CH4 be explained?
- 68. Chapter 20 Slide 68 Hybridization and sp3 Hybrid Orbitals 4 equivalent orbitals How can the bonding in CH4 be explained?
- 69. Chapter 20 Slide 69 Hybridization and sp3 Hybrid Orbitals
- 70. Chapter 20 Slide 70 Hybridization and sp3 Hybrid Orbitals
- 71. Chapter 20 Slide 71 Other Kinds of Hybrid Orbitals
- 72. Slide 72 Hybrid Orbitals in Coordinated Complexes We should look at magnetic property of the complex to see if they are high or low spin. Then we could decide whether they are using d2sp3 or sp3d2 hybrid
- 73. Chapter 20 Slide 73 The octahedral d2sp3 and sp3d2
- 74. Chapter 20 Slide 74 Square Planar geometry of four dsp2
- 75. Chapter 20 Slide 75 Bonding in Complexes: Valence Bond Theory Experimental results: High Spin
- 76. Chapter 20 Slide 76 Bonding in Complexes: Valence Bond Theory Experimental results: Low Spin
- 77. Chapter 20 Slide 77 High- and Low-Spin Complexes Low spin: Minimum number of unpaired electron High spin: Maxium number of unpaired electron, Paramagnetic Experimental results : High Spin [CoF6]3- [Co(CN)6]3-
- 78. Chapter 20 Slide 78 Crystal Field Theory Crystal Field Theory: Effect of charges of ligand on transition metal d-electrons. A model that views the bonding in complexes as arising from electrostatic interactions and considers the effect of the ligand charges on the energies of the metal ion d orbitals.
- 79. Chapter 20 Slide 79 Crystal Field Theory Directed at ligands Directed between ligands Octahedral Complexes
- 80. Chapter 20 Slide 80 Crystal Field Theory Octahedral Complexes
- 81. Chapter 20 Slide 81 Crystal Field Theory [Ni(X)6]2+ X=H2O, NH3, and ethylenediamine (en) Octahedral Complexes (red-violet)
- 83. Chapter 20 Slide 83 Crystal Field Theory [Ni(X)6]2+ X=H2O, NH3, and ethylenediamine (en) Octahedral Complexes The crystal field splitting changes depending on nature of the legand.
- 84. Chapter 20 Slide 84 The absorption maximum for the complex ion [Co(NH3)6]3+ occurs at 470 nm. What is the color of the complex and what is the crystal field splitting in kJ/mol? DE = hn hc l = = 4.23 x 10-19 J DE (kJ/mol) ? = 255 kJ/mol





![Slide 6
a)S [Ne]3s23p4
Using periodic table write Noble gas notation
for the following elements:
b)Fe
[Ar] 4s23d6
c)Se [Ar] 4s23d104p4
d)Gd [Xe]6s24f75d1
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s
< 4f< 5d< 6p< 7s< 5f< 6d< 7p](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-6-320.jpg)




![Chapter 20 Slide 11
Coordination
Compounds of Ni2+
[Ni(NH2C2H4NH2)3]+2
[Ni(NH3)6]+2](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-11-320.jpg)
![Chapter 20 Slide 12
Electron Configurations
Zn (Z = 30): [Ar] 3d10 4s2
Sc (Z = 21): [Ar] 3d1 4s2](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-12-320.jpg)




![Chapter 20 Slide 18
Poly DentateLigands
• EDTA4– is often used to treat
heavy metal poisoning such
as Hg2+, Pb2+, and Cd2+.
• EDTA4– bonds to Pb2+, which
is excreted by the kidneys as
[Pb(EDTA)]2–.](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-18-320.jpg)




![Chapter 20 Slide 24
What are the oxidation numbers of the metals in
K[Au(OH)4] and [Cr(NH3)6](NO3)3 ?
OH- has charge of -1
K+ has charge of +1
? Au + 1 + 4x(-1) = 0
Au = +3
NO3
- has charge of -1
NH3 has no charge
? Cr + 6x(0) + 3x(-1) = 0
Cr = +3](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-24-320.jpg)


![Chapter 20 Slide 27
Coordination Sphere
• Coordinate bond:
• Coordination Sphere: is the central metal and
surrounding ligands. The square brackets separate
the complex from counter ions such as SO4
2–.
H
H
H
H
H
H
H
H
+
H
H
H
H
Ag+
(aq) 2 Ag
+
[Ag(NH3)2]2 SO4
N N N](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-27-320.jpg)









![Chapter 20 Slide 37
Name the following Complexes:
Pt(
Tris(ethylenediamine)nickel(II)
IrCl(CO)(PPh3)2
Carbonylchlorobis(triphenylphosphine)iridium(I)
[Ni(NH2C2H4NH2)3]2+](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-37-320.jpg)
![Chapter 20 Slide 38
What is the systematic name of
[Cr(H2O)4Cl2]Cl ?
tetraaquadichlorochromium(III) chloride
Write the formula of
tris(ethylenediamine)cobalt(II) sulfate
[Co(en)3]SO4](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-38-320.jpg)
![Chapter 20 Slide 39
1. Constitutional Isomers: Have different bonds
among their constituent atoms.
• Ionization Isomers :
[Co(NH3)5Br]SO4 (violet compound with Co–Br bond),
[Co(NH3)5 SO4]Br (red compound with Co–SO4 bond).
• Linkage Isomers form when a ligand can bond through
two different donor atoms. Consider [Co(NH3)5NO2]2+
which is yellow with the Co–NO2 bond and red with the
Co–ONO bond.
Constitutional Isomerism](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-39-320.jpg)



![Chapter 20 Slide 43
• Geometric Isomers of [Co(NH3)4Cl2]Cl:
Isomers](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-43-320.jpg)












![Slide 57
Co
H3N
H3N NO2
NH3
NH3
NH3
2+
Co
H3N
H3N ONO
NH3
NH3
NH3
2+
sunlight
[Co(NH3)5Br]SO4 (violet),
[Co(NH3)5 SO4]Br (red ).
See next slide for Diastereoisomers](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-57-320.jpg)

![Chapter 20 Slide 59
Bonding in Complexes 01
• Bonding Theories attempt to account for the
color and magnetic properties
of transition metal complexes.
Ni2+ Cu2+
Zn2+
Co2+
•Solutions of [Ni(H2O)6]2+,
[Ni(NH3)6]2+, & [Ni(en)3]2+](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-59-320.jpg)
















![Chapter 20 Slide 77
High- and Low-Spin Complexes
Low spin: Minimum number of unpaired electron
High spin: Maxium number of unpaired electron, Paramagnetic
Experimental results : High Spin
[CoF6]3-
[Co(CN)6]3-](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-77-320.jpg)



![Chapter 20 Slide 81
Crystal Field Theory
[Ni(X)6]2+ X=H2O, NH3, and ethylenediamine (en)
Octahedral Complexes
(red-violet)](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-81-320.jpg)
![[Ti(H2O)6]3+
Chapter 20 Slide 82](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-82-320.jpg)
![Chapter 20 Slide 83
Crystal Field Theory
[Ni(X)6]2+ X=H2O, NH3, and ethylenediamine (en)
Octahedral Complexes
The crystal field splitting changes depending on nature
of the legand.](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-83-320.jpg)
![Chapter 20 Slide 84
The absorption maximum for the complex ion
[Co(NH3)6]3+ occurs at 470 nm. What is the color of
the complex and what is the crystal field splitting in
kJ/mol?
DE = hn
hc
l
= = 4.23 x 10-19 J
DE (kJ/mol) ?
= 255 kJ/mol](https://image.slidesharecdn.com/4910461-230823005138-d687354b/85/4910461-ppt-84-320.jpg)