7. evaluation of diagnostic test
- 1. Evaluation of Diagnostic Test Dr. A.P. Kulkarni MD, DPH, PhD, FIAPSM drapkulkarni@gmail.com
- 2. Lesson objectives At the end of the session the participants would be able to A.Enlist the indicators for evaluation of a diagnostic test B.Describe and calculate sensitivity, specificity & predictive power C.Describe the utility of ROC curve D.Describe the utility of Kappa Statistics
- 3. Indicators • Validity • Reproducibility • Acceptability • Feasibility : Validity Sensitivity Specificity Predictive Power Positive Test Negative Test
- 4. Gold standard • Universally accepted test • Validity indicators calculated with reference to gold standard
- 5. Test Result Disease (As diagnosed by golden test) Yes No Positive True + ve False + ve Negative False - ve True - ve
- 6. Test Result Disease Total Yes No Positive a b a + b Negative c d c + d Total a + c b+ d n
- 7. Test Disease Total Yes No + ve 90 10 100 -Ve 20 180 200 Total 110 190 300 Example
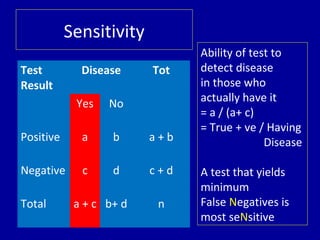
- 8. Test Result Disease Tot Yes No Positive a b a + b Negative c d c + d Total a + c b+ d n Sensitivity Ability of test to detect disease in those who actually have it = a / (a+ c) = True + ve / Having Disease A test that yields minimum False Negatives is most seNsitive
- 9. Test Disease Total Yes No + ve 90 10 100 -ve 20 180 200 Total 110 190 300 Example Sensitivity = 90 / 110 = 0.8182 =81.82 %
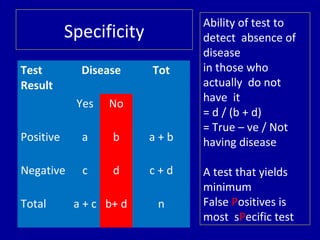
- 10. Test Result Disease Tot Yes No Positive a b a + b Negative c d c + d Total a + c b+ d n Specificity Ability of test to detect absence of disease in those who actually do not have it = d / (b + d) = True – ve / Not having disease A test that yields minimum False Positives is most sPecific test
- 11. Test Disease Total Yes No + ve 90 10 100 -ve 20 180 200 Total 110 190 300 Example Specificity = 180 / 190 = 0.9473 = 94.73 %
- 12. Test Result Disease Tot Yes No Positive a b a + b Negative c d c + d Total a + c b+ d n Predictive Power of Positive Test Probability that a person will have disease if test is positive = a / ( a + b ) = True + ve / Test + ve
- 13. Test Disease Total Yes No + ve 90 10 100 -ve 20 180 200 Total 110 190 300 Example Pr. Power (+ve Test) = 90 / 100 = 0.9000 = 90.00 %
- 14. Test Result Disease Tot Yes No Positive a b a + b Negative c d c + d Total a + c b+ d n Predictive Power of Negative Test Probability that a person will NOT have disease if test is negative = d/ ( c + d ) = True -ve / Test - ve Predictive power also depends on prevalence of disease
- 16. Test Disease Total Yes No + ve 90 10 100 -ve 20 180 200 Total 110 190 300 Example Pr. Power (-ve Test) = 180 / 200 = 0.9000 = 90.00 %
- 17. Reproducibility • Ability of test to give consistent results when repeated under similar conditions. Observer errors Errors in instrument / procedures Biological variation Improvement in reproducibility Training of personnel Standardization of procedures & instruments Multiple testing and averaging
- 18. Points To Remember • New test is always compared with established test (Golden Standard) • All measures are relative to that golden standard and would change if golden standard is changed • If different cut-offs are set, sensitivity / specificity changes ( if in one increases, the other decreases) • Observed indicators are subject to sampling variation
- 19. Points to remember 1. Validity( Syn.= accuracy): ability of measurement to be correct on an average. 2. Reproducibility( Syn.= precision, repeatability, reliability): ability of measurement to give same or similar result with repeated measurement 3. Economicity( Syn.= efficiency): extent to which the expenditures on the test in clinical and public health practice commensurate with the results 4. Acceptability, cost, ease of administration, technical ease.
- 20. Points to remember • Accuracy: How close the estimates of new test are to the truth. Truth is what GOLD STANDARD says. So, for accuracy, Gold standard is a must. • Reproducibility: How close are the repeated estimates of the new test to each other. So, for reproducibility, we must repeat the test in similar circumstances. Gold standard is NOT required here.
- 22. ROC Curve • Receiver Operating Characteristic Curves Uses 1. Deciding cut-off points 2. Comparing two tests Example • If fasting blood sugar (FBS) after 2 hrs is taken as a NEW test and its sensitivity / specificity is compared against GTT ( the golden test) • The sensitivity / specificity would differ at different cut-offs of FBS levels
- 23. ROC Curve- Example • Y axis : Sensitivity • X axis : (1- Specificity ) • Maximizing sensitivity corresponds to some large value on y-axis • Maximizing specificity corresponds to some small value on x-axis • Good first choice will be the one corresponding to upper left corner
- 24. ROC Curve- Area Under Curve (AUC) • An important measure of accuracy of the test • If AUC = 1, then curve consists of two lines Vertical: 0,0 to 0,1 and horizontal : 0,1 to 1,1 (Indicated by bold blue line) : The best, ideal • If AUC =0.5: A diagonal line results (0,0 to 1,1 Indicates a test that cannot discriminate between normal & abnormal
- 25. ROC Curve- Area Under Curve (AUC) • Softwares can calculate area under curve in a given case • Two or more tests can be compared statistically • May consist of measurements on same individuals (Paired Test) or on different individuals (Un-paired Test) • Test with higher AUC will be preferred .
- 26. Which Test Would You Select ? AreaUC = 0.918 95 % CI: 0.878, 0.958 AreaUC = 0.803 95% CI: 0.737, 0.870
- 27. What Cut Off Point Would You Select? +ve if => Sensitivity 1 - Specificity 64.0 1.0000 1.0000 67.5 1.0000 0.8837 72.5 1.0000 0.7791 77.5 1.0000 0.6628 82.5 0.9903 0.5581 87.5 0.9806 0.4535 92.5 0.9709 0.3488 97.5 0.9417 0.2674 102.5 0.8932 0.2093 107.5 0.8350 0.1628 +ve if => Sensitivity 1 - Specificity 112.5 0.7670 0.1279 117.5 0.6990 0.0930 122.5 0.6214 0.0698 127.5 0.5437 0.0465 135.0 0.4660 0.0233 142.5 0.3786 0.0116 147.5 0.2816 0.0116 152.5 0.1845 0.0116 157.5 0.0874 0.0116 161.0 0.0000 0.0000
- 28. Row +ve if => Sensitivity 1 - Specificity Specificity Sensitivity + Specificity 1 64.0 1.0000 1.0000 0 1.0000 2 67.5 1.0000 0.8837 0.1163 1.1163 3 72.5 1.0000 0.7791 0.2209 1.2209 4 77.5 1.0000 0.6628 0.3372 1.3372 5 82.5 0.9903 0.5581 0.4419 1.4322 6 87.5 0.9806 0.4535 0.5465 1.5271 7 92.5 0.9709 0.3488 0.6512 1.6220 8 97.5 0.9417 0.2674 0.7326 1.6743 9 102.5 0.8932 0.2093 0.7907 1.6839 10 107.5 0.8350 0.1628 0.8372 1.6722 11 112.5 0.7670 0.1279 0.8721 1.6391 12 117.5 0.6990 0.0930 0.907 1.6060 13 122.5 0.6214 0.0698 0.9302 1.5516 14 127.5 0.5437 0.0465 0.9535 1.4972 Identify the row where total of sensitivity and specificity is highest
- 29. Row +ve if => Sensitivity 1 - Specificity Specificity Sensitivity + Specificity 1 64.0 1.0000 1.0000 0 1.0000 2 67.5 1.0000 0.8837 0.1163 1.1163 3 72.5 1.0000 0.7791 0.2209 1.2209 4 77.5 1.0000 0.6628 0.3372 1.3372 5 82.5 0.9903 0.5581 0.4419 1.4322 6 87.5 0.9806 0.4535 0.5465 1.5271 7 92.5 0.9709 0.3488 0.6512 1.6220 8 97.5 0.9417 0.2674 0.7326 1.6743 9 102.5 0.8932 0.2093 0.7907 1.6839 10 107.5 0.8350 0.1628 0.8372 1.6722 11 112.5 0.7670 0.1279 0.8721 1.6391 12 117.5 0.6990 0.0930 0.907 1.6060 13 122.5 0.6214 0.0698 0.9302 1.5516 14 127.5 0.5437 0.0465 0.9535 1.4972 Identify the row where total of sensitivity and specificity is highest
- 30. Kappa Statistics • Is the agreement between two or test a chance occurrence or otherwise • “Significant agreement” means that element is ruled out
- 31. O=Observed frequency of agreement = a+d = 90 E1=Expected agreement (a) = (a+b)x((a+c)/N=12.24 E2=Expected agreement(d) =(c+d)x(b+d)/N= 42.64 E= E1+E2=54.88 New Test Gold Standard Total Disease Present Disease Absent Disease Present a (30) b (6) a+b (36) Disease Absent c (4) d (60) c+d (64) Total a+c (34) b+d (66) N (100)
- 32. New Test Gold Standard Total Disease Present Disease Absent Disease Present a (30) b (6) a+b (36) Disease Absent c (4) d (60) c+d (64) Total a+c (34) b+d (66) N (100) Kappa = [Obsv Agree – Exp Agree] / [Total-Exp Agree] = [O-E ] / [N-E] = [90-54.88] / [100-54.88] = 35.12 / 45.12 = 0.780
- 33. Interpretation of Kappa values K Interpretation < 0 No agreement 0 – 0.19 Negligible 0.20 – 0.39 Minimal agreement 0.40 – 0.59 Fair agreement 0.60 – 0.79 Good agreement 0.80 – 1.00 Excellent agreement 0.7783
- 35. Further Evaluation 1. Likelihood Ratio 2. Post Test Odds and Post Test Probability 3. ROC Curves • Predictive power of test is affected by prevalence of the disease • Can be used for combination of tests • Can be used for several levels of test
- 36. Further Evaluation • Pre Test Probability: Prevalence rate : Probability that a person will have the target disorder before the test is carried out • Pre Test Odds: The odds that the patient has the target disorder before the test is carried out (pre-test probability/ [1 - pre-test probability]).
- 37. Further Evaluation • Pre Test Probability: Prevalence rate : Probability that a person will have the target disorder before the test is carried out • Pre Test Odds: The odds that the patient has the target disorder before the test is carried out • Post Test Probability: The proportion of patients with that particular test result who have the target disorder • Post Test Odds: The odds that the patient is declared to have the target disorder after the test is carried out
- 38. Further Evaluation • Pre Test Probability: (PrTP) Prevalence rate : Persons with disorder ÷ Persons without disorder [Range: 0-1] • Pre Test Odds: (PrTO) = PrTP / (1- PrTP) [Range 0 to Infinity] • Post Test Probability: (PoTP) • Post Test Odds: (PoTO) Requires calculation of Likelihood Ratio
- 39. Further Evaluation • Likelihood Ratio Positive Test (LRP) LRP = Sensitivity / (1- Specificity) • Likelihood Ratio Negative Test (LRN) LRN = (1- Sensitivity) / Specificity • Post Test Probability Positive Test (PoTPP) (Probability that test +ve will have target disease) PoTPP = PrTP x LRP • Post Test Probability Negative Test (PoTPN) Probability that a test –ve will have target disease PoTPN = PrTP x LRN
- 40. Likelihood Ratio & Post Test Probability Test Disease Tota l Yes No + ve 90 10 100 -ve 20 180 200 Tota l 110 190 300 Likelihood Ratio (+ve test) = Sensitivity / (1-Specificity) = 0.8182 / (1- 0.9493) = 16.13 Post Test Probability + test = LHR+ / (1+LHR+) = 16.17 / (1+16.17) =0.9417 ( = 94.17 %) Probability that test positive will have disease is @ 94% Pre Test Probability (Prevalence) = 110 / 300 = 0.3666 ( 36.66%) Pretest Odds = 0.3666 / (1- 0.3666) = 0.5789
- 41. Likelihood Ratio & Post Test Probability Test Disease Tota l Yes No + ve 90 10 100 -ve 20 180 200 Tota l 110 190 300 Likelihood Ratio (-ve test) = (1-Sensitivity )/ Specificity = (1-0.8182) / (0.9493) = 0.1915 Post Test Probability -ve test = LHR v-ve / (1+LHR-ve) = 0.1915 / (1+0.1915) = 0.1607 (= 16.07%) Probability that test –ve will have disease is @ 16% ( Ideally this should be =0.0 % Pre Test Probability (Prevalence) = 110 / 300 = 0.3666 ( 36.66%) Pretest Odds = 0.3666 / (1- 0.3666) = 0.5789





























![New Test
Gold Standard Total
Disease Present Disease
Absent
Disease Present a (30) b (6) a+b (36)
Disease Absent c (4) d (60) c+d (64)
Total a+c (34) b+d (66) N (100)
Kappa = [Obsv Agree – Exp Agree] / [Total-Exp Agree]
= [O-E ] / [N-E]
= [90-54.88] / [100-54.88] = 35.12 / 45.12
= 0.780](https://image.slidesharecdn.com/7-180508134946/85/7-evaluation-of-diagnostic-test-32-320.jpg)



![Further Evaluation
• Pre Test Probability: Prevalence rate :
Probability that a person will have the target
disorder before the test is carried out
• Pre Test Odds: The odds that the patient has
the target disorder before the test is carried
out (pre-test probability/ [1 - pre-test
probability]).](https://image.slidesharecdn.com/7-180508134946/85/7-evaluation-of-diagnostic-test-36-320.jpg)

![Further Evaluation
• Pre Test Probability:
(PrTP) Prevalence rate :
Persons with disorder ÷
Persons without
disorder
[Range: 0-1]
• Pre Test Odds: (PrTO)
= PrTP / (1- PrTP)
[Range 0 to Infinity]
• Post Test Probability:
(PoTP)
• Post Test Odds: (PoTO)
Requires calculation of
Likelihood Ratio](https://image.slidesharecdn.com/7-180508134946/85/7-evaluation-of-diagnostic-test-38-320.jpg)


