Alkaloids
- 1. ALKALOIDS Prepared by Bhargav chand Pamula M. Pharmacy 1st year Department of pharmaceutical chemistry Guided by Dr. D. Ravi Sankar Reddy Assistant professor Acharya nagarjuna university college of pharmaceutical sciences
- 2. Contents : 1. Introducion 2. Classification 3. Isolation 4. Purification 5. Molecular modification and biological activity of alkaloids 6. Structural determination of alkaloids 7. Structural elucidation and stereochemistry of – ■ Ephedrine ■ Morphine ■ Ergot ■ Emetine ■ reserpine
- 3. Introduction : ■ Alkaloids are the basic,naturally occurring organic compounds that contains atleast one nitrogen atom. ■ The term alkaloid was proposed by the pharmacist w.Meissner in 1819. ■ The basic character of alkaloids is due to the presence of lone pair of electrons on nitrogen. ■ Alkaloids can neutral or acidic when the adjacent functional groups are electron releasing. ■ These are actually plant secondary metabolites produced as a chemical defensive mechanism against herbivore, parasites, microorganisms and viruses.
- 4. Classification : ■ Alkaloids are classified into- 1. Phenylethylamine alkaloids-ephedrine, adrenaline 2. Pyrrolidine alkaloids-Hygrine, cuskhygrine 3. Pyridine or piperidine alkaloids-Ricinine, coniine, Piperine 4. Pyridine-pyrrolidine alkaloids-Nicotine 5. Tropane alkaloids-Atropine, Cocaine, procaine, xylocaine 6. Quinoline alkaloids-Quinine, Cinchonine 7. Isoquinoline alkaloids-papaverine, narcotine, Berberine, emetine 8. Phenanthrene alkaloids-Morphine 9. Indole alkaloids-Ergotamine, reserpine,strychnine and brucine 10. Tropolone alkaloids-Colchicine
- 5. Alkaloids in food products : ■ Caffeine –present in coffee seeds ■ Theobromine-present in Cacao seeds ■ Theophylline and caffeine – present in tea leaves ■ Tomatine-present in tomatoes ■ Solanine- present in potatoes
- 6. Isolation of alkaloids : ■ Before performing isolation we should confirm whether alkaloids are present or not,for this the plant material is treated with various alkaloidal reagents such as tannic acid, picric acid, picolinic acid, perchloric acid, potassium mercuric iodide (Mayer’s reagent ),iodine dissolved in potassium iodide (Wagner’s reagent ),potassium bismuth iodide (Dragendorff’s reagent),phosphomolybdic acid (Sonneuschein’s reagent ),phosphotungstic acid (Scheibler’s reagent) with which alkaloids give either Precipitate or turbidity. ■ The dried and powdered plant material is first extracted with petroleum ether if it is rich in fat and then filtered for the removal of soluble fats.
- 7. ■ The residue is then extracted with methyl alcohol to remove cellulosic and other insoluble material and the filtrate so obtained is evaporated. ■ The evaporated mass is dissolved in water, acidified to pH 2 and finally steam distilled to remove methyl alcohol. ■ The dark residual solution is either allowed to stand for several days in refrigerator or heated with molten paraffin to remove suspended impurities. ■ The filtrate is extracted with ether or chloroform to remove water soluble non basic organic material and then steam distilled when steam volatile alkaloids are separated. ■ The rest of the alkaloid salts is made alkaline and again extracted with ether or chloroform and the ethereal layer obtained after extraction is evaporated to give crude alkaloids.
- 9. Purification of alkaloids : ■ Purification of alkaloids involves the separation of alkaloids from crude alkaloid and it can be carried out by – 1. Chramatographic techniques. 2. Gradient pH tetechnique. Chromatographic techniques: ■ Alkaloidal fractions can be Purified using different chramatographic techniques. ■ Column chromatography can be Performed by using silica gel or neutral alumina and chloroform or chloroform with Increased amounts of methanol are commonly usIng eluents. ■ Detection Is achieved by UV examination. ■ Gas chromatography is used in purification of volatile alkaloids.
- 10. ■ High performance liquid chromatography (HPLC) is the most effective technique for Seperation and also for quantitative determination of alkaloids e.g., solanum, papaver, cinchona, Datura,catharanthus alkaloids . ■ It is useful in Seperation of high molecular weight alkaloids. Gradient pH technique : ■ Though alkaloids are basic in nature, there are variations in the extent of basicity of various alkaloids of the same plant. ■ Depending on this character,crude alkaloidal mixture is dissolved in 2 percent tartaric acid solution and extracted with benzene so that first fraction contain very weakly basic alkaloids. ■ Then pH of the aqueous solution is increased gradually by 0.5 increments upto pH 9 and extraction is carried out at each pH level with organic solvent. ■ By this way alkaloids with different basicity are extracted, strong alkaloids extracted at the end.
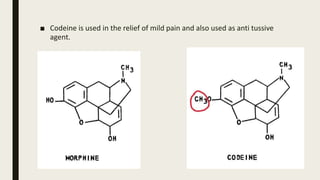
- 11. Molecular modification and biological activity of alkaloids : ■ Molecular modification is the chemical alteration of the known and previously characterized Lead compound for enhancing it’s usefulness as a drug. ■ Several alkaloids are modified molecularly for it’s clinical uses. Opium alkaloids : ■ Opium ,sun dried latex of the unripe fruit of Papaver Somniferum which contains atleast 23 alkaloids among them major alkaloids are – morphine, codeine, thebaine. ■ Morphine has been used as an important agent for the relief of pain. ■ This morphine is modified by methylation with methyl iodide to form monomethyl morphine also called as codeine.
- 12. ■ Codeine is used in the relief of mild pain and also used as anti tussive agent.
- 13. ■ Another modification of morphine is the esterification of two hydroxyl groups with acetic anhydride to give heroin. ■ Heroin is more potent than morphine without the respiratory depression effect. ■ Heroin crosses blood brain barrier more rapidly than morphine and once it reaches brain heroin hydrolyzes to morphine.
- 14. ■ Morphine is modified by the addition of methylene groups and the removal of two hydrogen atoms of hydroxyl groups to give thebaine. ■ It is neither an analgesic nor an anti tussive ,but resembles strychnine and brucine in its spinal convulsant properties.
- 15. Tropane alkaloids : ■ Atropine, scopolamine and cocaine are the tropane alkaloids having Tropane nucleus . ■ Atropine and scopolamine overlaps in pharmacodynamic activities but cocaine has uniqueness, being a anesthetic agent. ■ Procaine and lidocaine are local anesthetics derived from cocaine by molecular modification.
- 16. ■ Biological activity of some alkaloids are- ■ Tubocurarine - used as muscle relaxant in surgeries . ■ Vincristine, vinblastine – Used as chemotheratic agent in cancers. ■ Ergonovine – used as blood vessel constrictor. ■ Ephedrine – used in bronchial asthma. ■ Quinine – used as anti malarial drug. ■ Quinidine – used in arrythmias. ■ Colchicin – used to treat gout attacks.
- 17. General methods of structural determination of alkaloids : ■ Molecular structure of an alkaloid is determined according to the following steps- 1. First step in determining the structure of a pure alkaloid involves finding its molecular formula and optical rotatory power. 2. The presence of unsaturation in an alkaloid can be determined by the addition of bromine or halogen acids or by hydroxylation with dilute alkaline permanganate. Reduction may also used to show unsaturation and it can be done by several reagents like –lithium aluminum hydride and sodium borohydride. 3. Frequently an alkaloid is cleaved into simple fragments by hydrolysis with water,acid or alkali and the fragments so obtained are examined separately so, the structure of fragments can be easily established.
- 18. 4. Next step involves determining the functional nature of oxygen and nitrogen atoms either in molecule or in fragments. 5. Functional nature of oxygen : The oxygen atom may be present in the form of alcoholic or phenolic hydroxyl (-OH), methoxyl (-OCH3 ),acetoxyl (- OCOCH3), benzoxyl (-OCOC6H5) ,carboxyl (-COOH) or carbonyl (C=O)group. various oxygen functional groups can be characterized according to the following charecteristics- ■ Phenolic hydroxyl group (=C-OH) :presence of phenolic hydroxyl group is characterized by alkali solubility followed by reprecipitation by carbon dioxide. ■ Alcoholic hydroxyl group (-C-OH) :Presence of alcoholic hydroxyl group is generally indicated by its acylation reaction along with the negative tests for phenolic group.
- 19. ■ Carboxyl group (-COOH) :Presence of the carboxylic group is indicated by its solubility in weak bases like NaHCO3,NH3, esterification with alcohols. 6. Functional nature of nitrogen : The nitrogen atom may be present as secondary or tertiary. An alkaloid on distillation with aqueous potassium hydroxide generally gives methylamine, dimethylamine or trimethylamine indicates one, two or three alkyl groups attached to the nitrogen.lf ammonia is formed it indicates free amino group. 7. Estimation of C-methyl groups : C-methyl groups are quantitatively estimated by heating the compound with acidified potassium dichromate to form acetic acid which is distilled and estimated against strong base. 8. Degradation of alkaloids : it is the most important step in determining the structure of a compound Since it give certain products of well known structures and hence by knowing changes in degradation and structure of degraded products it will be easy to know structure of original molecule.
- 20. 9. Physical methods : physical techniques used for determining the structure of alkaloids are – – Infrared spectroscopy – Ultraviolet spectroscopy – X-ray analysis – NMR spectroscopy – Mass spectroscopy – Optical rotatory dispersion (ORD)
- 21. Ephedrine : ■ Ephedrine is an Ephedra alkaloid which has pronounced mydriatic action and astringent action .It is also used in the treatment of hay fever. Structural determination : 1. Its molecular formula is C10H15NO. 2. On treatment with nitrous acid, ephedrine gives nitroso compound indicating the presence of secondary amino group. 3. On benzoylation,it forms dibenzoyl derivative where one benzoyl group introduced at –NH group and other at –OH group. 4. On oxidation,ephedrine gives benzoic acid that is monosubstituted benzene. 5. Ephedrine when heated with hydrochloric acid forms methylamine and propiophenone.
- 22. Above reaction suggests either of the following two structures to ephedrine. 6. Ephedrine hydrochloride on heating,undergoes hydramine fission to form propiophenone, phenylacetone and methylamine.Since the hydramine fission is characteristic of compounds having C 6H 5CHOH.CH(NHR)CH3 group, ephedrine must be given structure 2.
- 23. 7. The above structure for ephedrine is supported by its Hofmann exhaustive methylation to sym-methylphenylethylene oxide. 8. The structure proposed by degradation is further confirmed by its synthesis and optical isomerism. ■ Racemic ephedrine is synthesised by the catalytic reduction of benzoylacetyl(1-phenylpropane-1,2-dione) in the presence of methamine in methanol solution.
- 24. ■ The Racemic ephedrine is resolved into optically active ephedrine by mandelic acid. Optical isomerism in ephedrine : ■ Since ephedrine has two dissimilar asymmetric carbon atoms,four optically active isomers are possible i.e.two enantiomeric pairs.
- 25. Morphine : ■ Morphine is the most important among the opium alkaloids and the other closely related alkaloids are codeine and thebaine. ■ These three alkaloids are commonly called as morphine alkaloids. Structural elucidation : 1. Its molecular formula is C17H19NO3. 2. Morphine takes up one mole of methyl iodide to form quaternary ammonium salt showing that nitrogen is present as tertiary one. 3. Morphine on acetylation or benzoylation gives the diacetyl(heroin) or dibenzoyl derivative indicating the presence of two hydroxyl groups. 4. By colouration with FeCl3 and solubility in alcoholic aqueous sodium hydroxide to form monosodium salt which is reconverted into morphine by passing through CO2. Hence one of the hydroxyl group must be phenolic one.
- 26. 5. On treatment with halogen acids,morphine forms monohalogeno product indicates the presence of alcoholic hydroxyl group. 6. From the unreactivity of the third oxygen atom and the degradation products of morphine it was concluded that the third oxygen atom is present as an ether linkage. 7. On catalytic reduction codeine gives isolated dihydro product ,C18H23O3N suggesting the presence of isolated ethylenic bond. 8. Morphine is brominated to a bromo derivative along with the evolution of a mole of hydrogen bromide which suggests morphine possesses a benzene nucleus. 9. Morphine on distillation with zinc dust gives Phenanthrene indicating the presence of benzene nucleus in morphine molecule.
- 27. 10. Codeine is treated with methyl iodide to form codeine methiodide,which on heating with alkali gives α-codeimethine. ■ Based on these changes correspond to Hofmann degradation of N- methylpiperidine the nitrogen atom must be present in a ring. 11. α-codeimethine on heating with alkali suffers a double bond shift to give isomeric β-codeimethine. When either of these isomers is treated with methyl iodide methylmorphenol is formed.This methylmorphenol when heated with hydrobromic acid gives morphenol which on reduction with sodium and alcohol gives morphol.
- 28. 12. Since morphol is obtained by the reduction of morphenol which on fusion with KOH affords 3,4,5-trihydroxyphenanthrene, the morphenol will have following structure. 13. Codeine methiodide and codeinone methiodide on heating separately with a mixture of Ac2O-AcONa gives 3-methoxy-4-acetoxyphenanthrene and 3-methoxy-4,6-diacetoxyphenanthrene. 14. As indicated by the fact that morphine forms monobromo derivative with bromine and monosodium salt with sodium hydroxide, it possesses only one benzenoid nucleus .
- 29. ■ Further,as ethylene is formed as one of the products during the exhaustive methylation of codeimethines and dimethylaminoethanol is formed. Thus a –CH2-CH2–Nme chain must be present in morphine. Further as it contains a double bond and a tertiary nitrogen atom the partial structure for morphine may be written as below.
- 30. 15. Codeine on oxidation with chromic acid gives some hydroxycodeine along with codeine the hydroxycodeine on Hofmann degradation gives a ketocodeimethine which on heating with Ac2O affords a methoxydiacetoxyphenanthrene. 16.Struture is further confirmed by X-ray analysis and course synthesis and is confirmed structure is.
- 31. Emetine: ■ Emetine is the principal alkaloid of the roots of cephaelis ipecacuanha. ■ In addition to this ipecacuanha consists cephaeline, psychotrine, o- methylpsychotrine and emetamine. In common these alkaloids are known as ipecacuanha alkaloids. Structural elucidation : 1. Its molecular formula is C29H40N2O4. 2. One of the two nitrogens is found to be present as secondary and the other as tertiary, but no N-methyl group is present. 3. On heating with hydroiodic acid ,Emetine yields four moles of methyl Iodide showing the presence of four groups of oxygen. 4. Oxidation of Emetine with permanganate in acetone gives m-hemipinic acid (1) and small amounts of 6,7-dimethoxyisoquinoline-1-carboxylic acid (2).
- 32. ■ Similarly, chromic acid oxidation of Emetine gives 4,5-dimethoxy- phthalonimide (3). These products indicates the presence of at least one 6,7- dimethoxyisoquinoline unit in Emetine. 5. Since the absorption spectra of Emetine does not resemble with that of fully aromatic isoquinoline like papaverine or the acid (2) but is similar to that of 1,2,3,4-tetrahydroisoquinoline derivatives .it must be derivative of tetrahydroisoquinoline.
- 33. 6. Ultraviolet absorption spectrum of emetine Closely resembles with that of tetrahydropapaverine showing that the emetine molecule must contain two O-dimethoxybenzene units. 7. Emetine on oxidation with alkaline permanganate gives first corydaldine(4), which removed and the residue is further oxidised to yield m-hemipinic acid(1).
- 34. 8. The position of oxide or carboxyl group in compounds (2)(3)(4) represents the point of attachment of the two tetrahydroisoquinoline units to the remainder molecule. Since one of the nitrogen atom in Emetine is tertiary ,the other nitrogen is also linked to rest of the molecule.Hence the partial structure of emetine may be represented as below.
- 35. 9. The nature of C4H7 fragment is established by the Hofmann degradation of emetine so as to remove completely the nitrogen atoms. 10. Going backwards from compound (4) to emetine, full carbon skeleton of emetine can therefore be depicted as-
- 36. ■ Now the only problem is to satisfy the third valency of one of the nitrogens so as to make it tertiary, for this there are three possibilities to give three different structures for emetine. 11. But R.Robinson proposed the correct structure for emetine as (8),since emetine has only one C-alkyl group the structure (9),(10)are discarded. 12. Finally the structure was proved by its synthesis.
- 37. Ergotamine : ■ Ergotamine is one of the several alkaloids isolated from the ergot or secale cornutum, product of a filamentous fungus claviceps purpurea which grows parasitically on rye and other grasses and cereals. ■ It is used in the treatment of migraine headaches. Structural elucidation : 1. The Molecular formula of ergotamine is C33H35N5O5 . 2. Ergotamine on alkaline hydrolysis gives d-lysergic acid as principal product and other products which depend upon individual ergot alkaloid .
- 38. ■ Hence the complete structure of ergotamine can be studied under two heads i.e. structure of lysergic acid and structure of non-lysergic acid moiety. 3. Structure of lysergic acid, C16H19NO2 : ■ By the usual tests lysergic acid is found to contain a carboxylic group ,non basic amino group of the type >NH,an NCH3 group and a double bond. ■ On potassium hydroxide fusion,dihydrolysergic acid yields 3,4- dimethylindole,1-methyl-5-aminonaphthalene and methylamine.
- 39. ■ This led to the following conclusion. (a) the presence of indole and naphthalene nuclei in lysergic acid. (b) the formation of CH3NH2 and 3,4-dimethylindole indicates that the nitrogen atom of 1-methyl-5-aminonaphthalene is not its own but derived from the Indole nucleus of the lysergic acid . ■ Lysergic acid on oxidation with HNO3 gives a tricarboxylic acid which on distillation with soda lime yields quinoline suggesting thereby that lysergic acid also possesses a quinoline nucleus .It indicates that lysergic acid must be tetracyclic of the following type.
- 40. ■ The comparison of uv spectra of lysergic and isolysergic acids with that of dihydrolysergic acid indicated that the additional double bond of lysergic acid and isolysergic acids is present in conjunction with the Indole system i.e.either between C9 and C10 or between C5 and C10. ■ Position of the carboxylic group in lysergic acid is found to be at C8 and it behaves as β-amino acid. ■ Finally the above structure of lysergic acid is confirmed by its synthesis. 4. Structure of non-lysergic acid moiety : ■ The hydrolysis of ergotamine to Lysergic acid,d-proline, phenylalanine and pyruvic acid indicates that the alkaloid contain peptide bonds which on hydrolysis gives amino acids.
- 41. ■ D-proline and phenylalanine are found to be present as such in the alkaloid, but the α-keto acid like pyruvic acid is not present as such since no free keto group is detected in the alkaloid. ■ Since the non-lysergic acid moiety neither contains a free carboxyl group nor a basic amino group,amino acids are present in the form of a cyclic structure. ■ Since mild alkaline hydrolysis gives lysergic acid –amide .instead of lysergic acid ,an acid-amide linkage is present between lysergic acid carboxyl and the α-hydroxy-α-amino acid moiety. ■ Treatment of dihydroergotamine with hydrazine gives dihydrolysergic acid hydrazine and propionyl-L-phenyl-alanyl-L-proline. ■ Mild hydrolysis of dihydroergotamine with one equivalent of alkali in alcohol gives lysergic acid and pyruvoyl-L-phenyl-alanyl-L-proline.
- 42. ■ The products of above two reactions indicate the following sequence of the amino acids in the non-lysergic acid moiety. Pyruvic acid – phenylalanine – proline ■ The products of above two reactions always contain a considerable quantity of diketopiperazine i.e. phenylalanineproline lactam Suggesting that in the alkaloid phenylalanine and proline units are linked with each other in a way to form six membered ring along with five membered ring. ■ Thus the peptide or non-lysergic acid portion of ergotamine may be written as
- 43. ■ Thus the complete structure of Ergotamine can be written as below -
- 44. Reserpine : ■ Reserpine is the main active principal of the Rauwolfia species. ■ It is used in the treatment of hypertension ,epilepsy. Structural elucidation : 1. The molecular formula of reserpine is C33H40N2O9 . 2. On heating with HI it gives 5 moles of methyl iodide indicating the presence of 5 methoxy groups. 3. As reserpine is a weak base, both the nitrogen atoms must be involved in the ring. Further as reserpine is lack of hydroxyl group,It forms acetyl derivative indicating the presence of an >NH group. It is further confirmed by I.R. spectra which reveals the presence of an Indole nucleus. Reserpine readily gives a methiodide ,so the second nitrogen atom must be tertiary.
- 45. 4. Reserpine upon alkaline hydrolysis gives methanol ,3,4,5-trimethoxy- benzoic acid and reserpic acid of composition C22H28N2O5 . ■ Since reserpine has no –COOH or –OH group the introduction of the two carboxyl acidic groups (one in 3,4,5-trimethoxybenzoic acid and another in reserpic acid) and two alcoholic groups (one in CH3OH and the other in reserpic acid) in the hydrolysis products suggest that reserpine is a diester.
- 46. 5. Structure of reserpic acid : ■ By the usual tests reserpic acid is found to possess two methoxy, one carboxylic, one secondary alcoholic, one >nh, and one tertiary amino groups. ■ On permanganate oxidation reserpic acid gives 4-methoxy-N-oxalyl anthranilic acid as one of the products confirming the presence of indole nucleus. ■ Reserpic acid on fusion with potash gives 5-hydroxyisophthalic acids. The hydroxyl and carboxyl groups in reserpic acid are meta to each other. ■ Selenium dehydrogenation of methyl reserpate affords a compound of the C19H16N2 as one of the principal product. This structure is called yobyrine.
- 47. ■ Yobyrine condenses with aldehydes suggesting the presence of a pyridine ring with a >CH2 substituent adjacent to nitrogen. It gives 3- ethylindole and isoquinoline on zinc dust distillation,phthalic acid on permanganate oxidation, and o-toluic acid on chronic oxidation.
- 48. ■ All these reactions suggest the following structure to yobyrine. ■ From the above points we know that one of the methoxy group is in the m-position to the –NH group of indole. i.e.on C11 .Presence of carboxyl group at C16 is indicated by the dehydrogenation of reserpic acid with selenium to 11-hydroxy-16-methylyobyrine.
- 49. ■ We also know that acidic and hydroxy groups are meta to each other the hydroxy group in reserpic acid must be present on C18. ■ The second methoxy group is assigned to the position 17.
- 50. ■ On the basis of reserpic acid structure, reserpine has been assigned the following structure. ■ The above structure of the reserpine has been proved by its synthesis.