An Environmental Trifecta
- 1. Coupling Desalination with Carbon Mineralization to eliminate CO2 and brine disposal, store energy, and reduce salination of groundwater John R. Hoaglund, III, Ph.D. john@h2o-c.com (814) 574-2649 Carbon Negative Water Solutions, LLC www.h2o-c.com
- 3. Overview Solutes from desalination “concentrate” (leftover salt) are used as a substrate to capture CO2, SOx, and NOx. Brine discharge is eliminated. Instead … Brine and water resources produce commodities: 1. Minerals: “mined” from solution, inc. a de-icing agent 2. Gaseous: including hydrogen for energy storage Freshwater production is increased from: 1. the remaining solvent leftover from solute extraction 2. stack capture of the water from the combustion of the hydrocarbon fuel (with water treatment onsite). 3. combustion or electrolytic oxidation of the hydrogen
- 4. How did Earth lower CO2 levels? CO2 in Archean atmosphere was at least 100 times higher! [4% versus 0.04% today (versus .03% in 1980)] Photosynthetic blue-green algae raised O2 levels, eventually oxidizing methane and excess Fe minerals, enough to oxygenate atmosphere ~ 2.3 Ba … Algae consumed the CO2 … but that’s only part of the story. Photosynthesis = respiration + decay Reason why plant-based carbon offsets provide only temporary carbon sequestration. Must bury the carbon. Geologic carbon deposits: Coal, oil, gases, carbonates
- 5. How did geologic carbon deposits form? Coal: burial of coal forests (later in Earth history) Clathrate: microbial reduction of deep marine CO2 Oil and related gas: burial of marine organic carbon, including algae Carbonates associated with blue-green algae (procaryotic cynanobacteria) removed the CO2 from Earth's original atmosphere. Stromatolites!
- 6. Photosynthesis associated carbonate sedimentation Stromatolites--fossilized blue-green algal mats--in 2.1 Ga Kona Formation dolomitic carbonates near Marquette,Michigan.
- 7. Natural carbonate formation: Acid is consumed (or base is added: H+ + OH- => H2O) Increasing pH increases total dissolved inorganic carbon in solution: Increasing pH stabilizes bicarbonate (HCO3 -)and carbonate (CO3 2-) in solution. Rising alkalinity splits between … hydroxide (OH-) base component bicarbonate (HCO3 -) and carbonate (CO3 2-) buffering components. H2O + CO2 <==> H2CO3 <==> H+ + HCO3 - HCO3 - <==> H+ + CO3 2-
- 8. Natural carbonate formation: H2O + CO2 <==> H2CO3 <==> H+ + HCO3 - HCO3 - <==> H+ + CO3 2-After Stumm and Morgan, 1981, Aquatic Chemistry Changing alkalinity components in a closed system of constant Ct with pH changing from the addition of base in a titration OH- HCO3 - CO3 2-
- 9. Natural carbonate formation: What consumes acid in some natural systems? Three examples: 1. Human respiration (a cycle) In cells: hemoglobin exchanges O2 for metabolic acid, while metabolic CO2 goes into blood as HCO3 In lungs: HCO3 neutralizes the acid into CO2 that is exhaled, enabling hemoglobin to re-uptake oxygen. H2O + CO2 <==> H2CO3 <==> H+ + HCO3 - HCO3 - <==> H+ + CO3 2-
- 10. Natural carbonate formation: What consumes acid in some natural systems? Three examples: 2. Chemical weathering: rocks are usually bases or release bases when reacted with acid. Carbonate rocks: CaCO3 + 2H+ => Ca2+ + H2O + Hydrolysis of Feldspar to clay: 4KAISi3O8 + 4H+ + 2H20 → Al4Si4O10(OH)8+ 8SiO2 + 4K+ H2O + CO2 <==> H2CO3 <==> H+ + HCO3 - HCO3 - <==> H+ + CO3 2- CO2
- 11. Natural carbonate formation: What consumes acid in some natural systems? Three examples 3. Ocean: photosynthesis uptakes acid as well as carbon, phosphorous and nitrate in even proportions The Redfield ratio. (C : N : P : H) = (106 : 16 : 1 : 18 ) 106CO2 + 16NO3 - + HPO4 2- + 122H2O + 18H+ C106H263O110N16P1 + 138O2 H2O + CO2 <==> H2CO3 <==> H+ + HCO3 - HCO3 - <==> H+ + CO3 2-
- 12. Alkalinity components in an open system exposed to constant atmospheric CO2 = 315 ppm After Stumm and Morgan, 1981, Aquatic Chemistry
- 13. Alkalinity components in an open system exposed to constant atmospheric CO2 = 315 ppm After Stumm and Morgan, 1981, Aquatic Chemistry
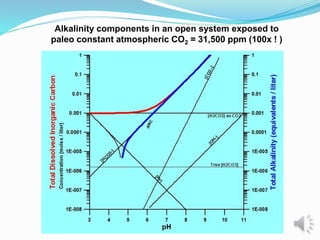
- 14. Alkalinity components in an open system exposed to paleo constant atmospheric CO2 = 31,500 ppm (100x ! )
- 15. Alkalinity components in an open system exposed to paleo constant atmospheric CO2 = 31,500 ppm (100x ! )
- 16. Natural carbonate formation: Can we mimic this industrially?
- 17. Natural carbonate formation: Yes, with brine electrolysis!
- 18. 2000: Moved to Penn State for coupled climatological / hydrological modeling. Al Gore is going to be in the White House. Life is set. 2002 Read The Hydrogen Economy by Jeremy Rifkin Hydrogen electrolyzed for trucks converted to run gas. 2002 Attended Penn State Hydrogen Conference The new hot topic: hydrogen … and fuel cells ICE versus fuel cells: Q: “Why not just burn hydrogen in an ICE?” A: NOx + heat waste I raised concern about freshwater demands. Water Electrolysis Hydrogen Production [2002]
- 19. Water Electrolysis Hydrogen Production [2002] 90% of hydrogen is from the steam reforming of natural gas. Solves nothing: CH4 + 2H2O => CO2 + 4H2 10% electrolytic hydrogen from fresh water 2H2O + e- => 2H2 + O2 Requires freshwater. Resource competition? “Freshwater exiting a fuel cell is from freshwater entering an elecrolyzer.” Hoaglund, J.R., C. Hochgraf, and T. Bohn, 2003. The hydrogen effluent. Ground Water v 41, n 4. p. 404-405. Why? “Most efficient production uses a KOH solution.”
- 20. Compliments of a 2002 phone call with Hydrogenics Anode oxidation (“current into device” = electrons out) 2H2O + 4KOH ==> O2 + 4H+ + 4OH- + 4K+ + 4e- ==> O2 + 4H2O + 4K+ + 4e- Cathode reduction (electrons in): 4H2O + 4K+ + 4e- ==> 2H2 + 4OH- + 4K+ ==>2H2 + 4KOH KOH is recycled. Water Electrolysis Hydrogen Production [2002]
- 21. On to carbon sequestration … EPRI interview question, 2006 Interested in my background in the Michigan Basin for CO2 sequestration in basin structures and brine CO2 is not only an asphyxiant, but at high concentrations, a lethal gas “Would you be comfortable handling the public relations aspects of an underground injection proposal?” Carbon sequestration processes are industrial processes (not necessarily environmentally friendly)
- 22. Going to California Perchlorate work, 2007-2010 2NaCl + 6H2O => NaClO4 + HOCl (oxidation half) + 5H2 + NaOH (reduction half) DOW Chemical’s earliest products from the electrolysis of Michigan Basin brines in Midland, MI. DOW must assure NaOH product is air-tight to prevent atmospheric contamination? Why? NaOH (aq) + CO2 (g) ==> NaHCO3 (s) baking soda
- 23. 2NaCl (aq) + 2H2O (aq) + 2CO2 (g) + e- => H2 (g) + Cl2 (g) + 2NaHCO3 (s) 1) Brine Electrolysis 2NaCl (aq) + 2H2O (aq) + e- => H2 (g) + Cl2 (g) + 2NaOH (aq) Going to California Putting it together, 2010 Salt: “An ionic compound that results from the neutralization of an acid with a base.” Electrolysis “undoes” the salt back into base NaOH … then 2) CO2 Aeration 2CO2 (g) + 2NaOH (aq) => 2NaHCO3 (s)
- 24. Alkalinity components in an open system exposed to paleo constant atmospheric CO2 = 31,500 ppm (100x ! )
- 25. 2NaCl (aq) + 2H2O (aq) + 2CO2 (g) + e- => H2 (g) + Cl2 (g) + 2NaHCO3 (s) 1) Brine Electrolysis 2NaCl (aq) + 2H2O (aq) + e- => H2 (g) + Cl2 (g) + 2NaOH (aq) 2) CO2 Aeration 2CO2 (g) + 2NaOH (aq) => 2NaHCO3 (s) Going to California Putting it together, 2010 Was anybody doing this? • J. Stolaroff: Use of NaOH sprays to stack capture CO2 • K. Lackner: Capturing atmospheric CO2 using NaOH
- 26. Going to California AES power / Poseidon desal
- 27. Original proposal to take 130 mega-gallons per day (MGD) producing 65 MGD freshwater and returning 65 MGD brine at double seawater conc: 70,000 ppm On the books since 2002 Variety of economic and environmental controversies. Approved by City of Huntington Beach, needs Orange Co Water District (OCWD) customer, awaiting final approval by CA Coastal Commission CA Coastal Commission requires GHG mitigation plan AB-32 regulates CO2 in excess of 25,000 metric tons/yr Going to California AES power / Poseidon desal
- 28. Two biggest environmental issues: brine discharge and CO2 Plan for brine: Ocean discharge of TDS. How much? ( Q )( r )( C ) = 17,655 metric tons / day Q = 65 MGD = 246,069 m3/day of brine r = 1025 kg/m3 solution density C = (70,000 parts / 1,000,000 parts) = 7% = twice seawater NaCl = 85% of ocean TDS = 15,009 metric tons / day Plan for CO2: Carbon offsetting by buying wetlands. Going to California AES power / Poseidon desal
- 29. Two biggest environmental issues: brine discharge and CO2 Plan for CO2: Carbon offsetting by buying wetlands. How much CO2 is Poseidon “indirectly emitting” ? Q = 65 MGD = 246,069 m3/day freshwater 4 kWatt-hrs/m3 seawater desal by reverse osmosis (RO) 0.55 kg CO2 per kWatt-hr (US EIA natural gas rate) 541 metric tons/day liability (198 k/yr vs. reg’s 25 k/yr) AES total CO2 is 11,933 metric tons/day (541 is ~ 4.5%) 904 MW plant produces 21,616,000 kWatt-hr /day Going to California AES power / Poseidon desal
- 30. 2010 Eureka! 2NaCl (aq) + 2H2O (aq) + 2CO2 (g) + e- => H2 (g) + Cl2 (g) + 2NaHCO3 (s) Spoke with Carnegie Mellon physical chemist: DOW looked into this and didn’t pursue it. Not enough market for chlorine products. Energy penalty is too high. “You’re talking about the Skyonic process.” Use brine electrolysis with CO2 aeration to sequester CO2, make H2, and eliminate brine discharge
- 31. 2010: Carbon Negative 2NaCl (aq) + 2H2O (aq) + 2CO2 (g) + e- => H2 (g) + Cl2 (g) + 2NaHCO3 (s) Called Skyonic: Had just completed demo scale Won $28 million DOE grant for project scale. Currently capturing 75,000 metric tons off of cement plant in San Antonio, Texas (Capitol Aggregates). Where do you get your salt? “We mix it.” !!!!!!!!!! Couple it with desalination !!!!!!!!!!
- 32. Budget: Salt 2NaCl (aq) + 2H2O (aq) + 2CO2 (g) + e- => H2 (g) + Cl2 (g) + 2NaHCO3 (s) There’s enough salt NaCl salt and CO2 are mole for mole; 1.33 mass for mass AES plant CO2 total mass is 11,933 metric tons per day Poseidon NaCl mass is 15,009 metric tons per day CO2 equivalent mass is 11,301 metric tons per day A little bit short on needed NaCl mass, but … … other salts available in TDS of 17,655 metric tons per day
- 33. Budget: Water 2NaCl (aq) + 2H2O (aq) + 2CO2 (g) + e- => H2 (g) + Cl2 (g) + 2NaHCO3 (s) You get more water! The other 65 MGD with solutes removed!! 2.6 MGD from CH4 + 2O2 => CO2 + 2H2O AES plant CO2 total mass is 11,933 metric tons per day H2O / CO2 GMW equivalent ~0.41 metric tons 2 for 1 moles yields 9,769 metric tons water per day Water treatment is onsite! Water from H2 oxidation relatively insignificant. Best to use H2 to convert Cl2 to HCl?
- 34. Budget: The Energy Penalty 2NaCl (aq) + 2H2O (aq) + 2CO2 (g) + e- => H2 (g) + Cl2 (g) + 2NaHCO3 (s) Step 1) Electrolysis is the energy intensive step 2NaCl + 2H2O + e- => H2 + Cl2 + 2NaOH DG = +422.66 kJ/mol 15,846 metric tons NaCl => 15,874 Mwatt-hr / day 73% of plant output But that’s at STP. Higher temps, lower activation E Some energy back from H2 … and commodities Step 2) Aeration of CO2 releases heat energy DG = -38.5 kJ/mol
- 35. Budget: Kinetics 2NaCl (aq) + 2H2O (aq) + 2CO2 (g) + e- => H2 (g) + Cl2 (g) + 2NaHCO3 (s) Step 1) Electrolysis is the rate limiting step 2NaCl + 2H2O + e- => H2 + Cl2 + 2NaOH Technology is scalable. Purpose of project scale application Step 2) Aeration of CO2 is fast NaOH (aq) + CO2 (g) => NaHCO3
- 36. Algae biofuel carbon recycling 1. Algae: photosynthesis uptakes acid as well as carbon, phosphorous and nitrate in even proportions The Redfield ratio. (C : N : P : H) = (106 : 16 : 1 : 18 ) 106CO2 + 16NO3 - + HPO4 2- + 122H2O + 18H+ C106H263O110N16P1 + 138O2 2. Algae kinetics is too slow to keep up with stack output 3. NaHCO3 is used to store / deliver CO2 for algae NaHCO3 + HCl NaCl + H2O + CO2 4. Recycles salt: NaCl
- 37. Chlorine Sequestration? Demand? Skyonic claims to be able to produce a desired proportion of chlorine products: Cl2, HCl, HOCl, NaOCl (bleach), ClOx Hydrochloric acid is the easiest to dispose of by Reacting with native metal: 6HCl + 2Al => 3H2 + 2AlCl3 Dissolving serpentine rocks into magnesium chlorides. Magnesium chloride used in another carbon sequestration method developed by Klaus Lackner (now at ASU) to produce magnesium carbonates. Reactions of Cl2 with H2 returns chlorine to chloride as HCl
- 38. A Lack of divalent cations … Ca and Mg form more stable carbonates but are not in sufficient enough quantity in seawater 1) Calera: combined CO2 and ocean salt to form CaPO4 cement. 2) Klaus Lackner’s solution for this: Serpentine Mg3(Si2O5)(OH)4 ore is used as the source for Mg cation. Process produces Mg(OH)2 reacted with CO2 to produce magnesite, MgCO3 Requires serpentine ore dissolved by hydrochloric acid (HCl) to form MgCl2 Reaction summary: Mg3(Si2O5)(OH)4 + 6HCl ==> 3MgCl2 + 2SiO2 + 5H2O 3MgCl2 + 6H2O ==> 3Mg(OH)2 + 6HCl (brine electrolysis step) 3Mg(OH)2 + 3CO2 ==> 3MgCO3 + 3H2O (CO2 aeration step) HCl is recycled Therefore no salt consumption (no advantage for desalination)
- 39. Conclusions Solutes from desalination “concentrate” (leftover salt) can be used as a substrate to capture CO2 The process combines brine electrolysis with CO2 aeration to form carbonate minerals Can be combined with algae for biofuel C recycling Brine discharge is eliminated, producing products Freshwater production is increased from: 1. the remaining solvent leftover from solute extraction 2. stack capture of the water from the combustion of the hydrocarbon fuel (with water treatment onsite). 3. combustion or electrolytic oxidation of the hydrogen
- 40. Conclusions NaHCO3 to replace NaCl as a de-icing agent NaCl Na+ + Cl- vs NaHCO3 Na+ + HCO3 - Na+ reacts with soil minerals but Cl- is conserved. In regions using road salt, Cl- levels increase at twice the rate of Na+ Instead, bicarbonate ( HCO3 - ) is beneficial, increasing groundwater alkalinity
- 41. Conclusions Energy Penalty is high There are industrial hazards (as with any C-seq) Lack of market demand may require chlorine sequestration Other techniques can create more stable carbonates, but they recycle salt and /or use ores. Desal: It’s not just for oceans anymore! There are related jobs for water professionals




![How did Earth lower CO2 levels?
CO2 in Archean atmosphere was at least 100 times higher!
[4% versus 0.04% today (versus .03% in 1980)]
Photosynthetic blue-green algae raised O2 levels,
eventually oxidizing methane and excess Fe minerals,
enough to oxygenate atmosphere ~ 2.3 Ba …
Algae consumed the CO2 … but that’s only part of the story.
Photosynthesis = respiration + decay
Reason why plant-based carbon offsets provide only
temporary carbon sequestration.
Must bury the carbon.
Geologic carbon deposits: Coal, oil, gases, carbonates](https://image.slidesharecdn.com/ngwacarboncapturehydrogenmineralwater-190724075147/85/An-Environmental-Trifecta-4-320.jpg)












![ 2000: Moved to Penn State for coupled climatological
/ hydrological modeling. Al Gore is going to be in the
White House. Life is set.
2002 Read The Hydrogen Economy by Jeremy Rifkin
Hydrogen electrolyzed for trucks converted to run gas.
2002 Attended Penn State Hydrogen Conference
The new hot topic: hydrogen … and fuel cells
ICE versus fuel cells: Q: “Why not just burn hydrogen
in an ICE?” A: NOx + heat waste
I raised concern about freshwater demands.
Water Electrolysis
Hydrogen Production [2002]](https://image.slidesharecdn.com/ngwacarboncapturehydrogenmineralwater-190724075147/85/An-Environmental-Trifecta-18-320.jpg)
![Water Electrolysis
Hydrogen Production [2002]
90% of hydrogen is from the steam reforming of
natural gas.
Solves nothing: CH4 + 2H2O => CO2 + 4H2
10% electrolytic hydrogen from fresh water
2H2O + e- => 2H2 + O2
Requires freshwater. Resource competition?
“Freshwater exiting a fuel cell is from freshwater entering an
elecrolyzer.”
Hoaglund, J.R., C. Hochgraf, and T. Bohn, 2003. The hydrogen effluent. Ground Water
v 41, n 4. p. 404-405.
Why? “Most efficient production uses a KOH solution.”](https://image.slidesharecdn.com/ngwacarboncapturehydrogenmineralwater-190724075147/85/An-Environmental-Trifecta-19-320.jpg)
![Compliments of a 2002 phone call with Hydrogenics
Anode oxidation (“current into device” = electrons out)
2H2O + 4KOH ==> O2 + 4H+ + 4OH- + 4K+ + 4e-
==> O2 + 4H2O + 4K+ + 4e-
Cathode reduction (electrons in):
4H2O + 4K+ + 4e- ==> 2H2 + 4OH- + 4K+
==>2H2 + 4KOH
KOH is recycled.
Water Electrolysis
Hydrogen Production [2002]](https://image.slidesharecdn.com/ngwacarboncapturehydrogenmineralwater-190724075147/85/An-Environmental-Trifecta-20-320.jpg)




















