Antifungal Susceptibility Test
- 1. ANTIFUNGAL SUSCEPTIBILITY Presenter : Dr Abhijit Kumar Prasad PGT, 2nd Year Moderator : Dr W. V. Lyngdoh Associate Professor Department of Microbiology
- 2. HISTORY • Antifungal susceptibility testing was not relevant until the introduction in the 1950s of amphotericin B • Nearly 30 years after the discovery of the first antibacterial agents, antifungal susceptibility testing lay fallow for many years • While not all fungal infections responded to amphotericin B, there were no alternatives • With the development of Flucytosine and Azole antifungal agents the differences within and between species started to become apparent
- 3. WHY DO WE NEED? • Increase in incidence of immuno-supressive states • Increasing incidence of invasive mycosis and life threatening infections as a significant public health issue • Emerging resistance • Correlate with in vivo activity and predict the likely outcome of therapy • Provide a reliable measure of the relative activities of two or more antifungal agents
- 4. Continued. . . • Growing concern about a shortage of effective antifungal agents and an increase in the resistance of fungal pathogens to the existing agents • Among the invasive mycoses, none is more important or common than candidiasis • Candidiasis, specifically candidemia, has been shown in numerous studies to be the most frequent mycotic infection in hospitalized patients and is associated with a significant attributable mortality and excess length of hospital stay
- 5. SURVIELLANCE PROGRAMME YEAR No. OF ISOLATE IN BSI % of Total Isolates % OF FLUCONAZOLE RESISTANCE C. albicans C. glabrata C. parapsilopsis C. tropicalis CDC 1998-2K 944 45 1 24 10 13 0 12 6 SWEDEN 1994-98 233 0 40 15 0 QUEBEC 1996-98 442 1 9 0 0 SCOPE 1997-2k 934 53 1 20 7 10 0 12 1 EIEIO 1999-01 254 58 0 20 10 7 0 11 0 JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 2002, p. 3551–3557 Vol. 40, No. 10 0095-1137/02/$04.000 DOI: 10.1128/JCM.40.10.3551–3557.2002 American Society for Microbiology. All Rights Reserved.
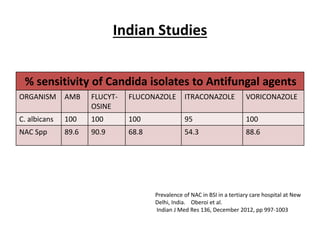
- 6. Indian Studies % sensitivity of Candida isolates to Antifungal agents ORGANISM AMB FLUCYT- OSINE FLUCONAZOLE ITRACONAZOLE VORICONAZOLE C. albicans 100 100 100 95 100 NAC Spp 89.6 90.9 68.8 54.3 88.6 Prevalence of NAC in BSI in a tertiary care hospital at New Delhi, India. Oberoi et al. Indian J Med Res 136, December 2012, pp 997-1003
- 7. ANTIFUNGAL AGENTS Class Generic Name & Available formulation Mechanism of action Azoles Imidazoles Triazoles Imidazoles Ketoconazole (oral tab/ shampoo) Miconazole (topical/oral) Econazole,Bifonazole,Ticonazole Triazoles Fluconazole (IV,oral susp/tab) Itraconazole (oral cap/Sol) Voriconazole (IV,oral tab) Posaconazole (Oral suspension) Interferes with ergosterol synthesis via inhibition of CYP dependent 14α demythylase, required for conversion of lanosterol to ergosterol
- 8. ANTIFUNGAL AGENTS Class Generic Name& Available formulation Mechanism of action Allylamines Terbinafine (oral, topical) Naftifine (topical) Butenafine (topical) Inhibit squalene epoxidase required for ergosterol formation Polyenes Amp B deoxycholate (IV, Oral sol) Amp B liposomal(IV) Amp B lipid complex(IV) Amp B colloidal disp(IV) Nystatin (topical) Pimaricin( Eye oint) Hamycin(Powder) Binds to ergosterol m& creates pores, leading to depolarization of the membrane & cell leakage Griseofulvin • Griseofulvin –Insoluble (oral) Acts by inhibiting microtubule function in dermatophytes
- 9. ANTIFUNGAL AGENT Class Generic Name& Available formulation Mechanism of action Pyrimidine Flucytosine ( 5FC) Oral Converted by Cytosine deaminase to 5- FU, an inhibitor of Thymidylate synthase, thus blocking nucleic acid synthesis Echinocandins Caspofungin (IV) Micafungin(IV) Anidulafungin(IV) Inhibit enzyme Glucan synthase which in turn inhibits formation of β1,3 Glycan synthesis, a component of fungal cell wall leading to increase cell wall permeability and lysis of the cell Thiocarbamates Tolnafate (topical) Nikkomycins – Inhibit Chitin synthesis, important component of cell wall
- 10. ANTIFUNGAL SUSCEPTIBILITY TESTS 1) BROTH DILUTION ANTIFUNGAL SUSCEPTIBILITY TEST 2) DISK DIFFUSION ANTIFUNGAL SUSCEPTIBITY TEST 3) E-TEST 4) VITEK
- 11. BROTH DILUTION ANTIFUNGAL SUSCEPTIBILITY TESTING FOR YEASTS (CLSI M27/A3) • Synthetic Media RPMI 1640 buffered with MOPS (Morpholine propanesulfonic acid) for Candida spp Yeast Nitrogen Broth for Cryptococcus neoformans • Antifungal agents Obtained commercially as pure salt Amphotericin-B, 5-Fluorocytosine, Azoles such as Fluconazole, Itraconazole, Voriconazole, Ketoconazole, Posaconazole, Ravuconazole Pharmaceutical preparations of these agents is not recommended
- 12. Preparation of Stock Solution • Water soluble antifungal agent - Fluconazole,5- fluorocytosine, Caspofungin, Micafungin are prepared in sterile distilled water • Water insoluble antifungal agent - amphotericin B, itraconazole, ketoconazole, posaconazole, ravuconazole, and voriconazole are prepared in dimethyl sulfoxide (DMSO) • Other solvents used are Ethyl Alcohol, Polyethylene glycol Carboxy methyl cellulose
- 13. Weighing of Antifungal powder • Weight (mg) = Volume (ml) x Conc (µg/ml) (FORMULA 1) Assay potency (µg/mg) Q) What amount of antifungal powder is to be weighed to prepare 100 ml of the stock solution containing 1280 /ml of antifungal agent with antifungal that has a potency of 750 µg/mg ? • Weight (mg) = 100 ml x 1280 µg/ml = 170.7 mg 750 µg/mg
- 14. Continued. . . “Because it is advisable to weigh a portion of the powder in excess of that required, powder was deposited on the balance until 182.6 mg was reached” – CLSI • Volume (ml) = Wt (mg) x Assay potency (µg/mg ) (Formula 2) Conc (µg/ml) • Volume (ml) = 182.6 mg x 750 µg/mg = 107ml 1280 µg/ml
- 15. Continued. . . • Stock solution are to be prepared at 10 times the highest concentration to be tested • Drugs dissolved in solvent are to be prepared at 100 times higher than the desired concentration to be tested • Each intermediate then should be further diluted to final strength in the test medium • Reason - Avoids dilution artifact that result from precipitation of compound with low solubility in aqueous media • Storage - at – 60°C or below but never at temperature greater than – 20°C, 6 months
- 16. Concentration of the drug to be tested • Concentration to be tested should encompass the breakpoint concentration & the expected results for the quality control strains • Based on previous studies, the following drug concentration ranges is to be used – Amphotericin B, 0.0313 to 16 ꭒg/ml Flucytosine, 0.125 to 64 ꭒg/ml Ketoconazole, 0.0313 to 16 ꭒg/ml Itraconazole & new triazoles 0.0313 to 16 ꭒg/ml Fluconazole, 0.125 to 64 ꭒg/ml
- 19. PROCEDURE • Inoculum preparation All organisms should be subcultured onto SDA or PDA Incubated at 35°C for 24 / 48 Hr Inoculum should be prepared by picking five well-isolated colonies of 1 mm in diameter Suspend the organisms in 5 ml of sterile saline Suspension equal to a 0.5 McFarland standard Working suspension is prepared by adding 0.1 ml of the suspension from above to 9.9 ml of RPMI Further diluted to 1:20 with RPMI to give a concentration of 5 x 102 to 2.5 x 103 cells / ml
- 20. Test inoculation and incubation (macrodilution method) • Add 0.1 ml of each antifungal (intermediate concentration for water-soluble drugs and for water insoluble drug intermediate concentration diluted to 1:10) to a sterile 12- by 75-mm test tube • Growth control tube - 0.1 ml of drug diluent without the antifungal agent • Second tube of drug diluent - Uninoculated to serve as a sterility control • Within 15 min of inoculum preparation, 0.9 ml of the inoculum is added to each tube in the series (Except sterility control) bringing antifungal agents to the final concentration • Incubation – 35°C for 46 to 50 h (Candida spp)
- 21. Reading Results • Amount of growth in each tube is compared to that of the growth control • For amphotericin B, the MIC is read as the lowest drug concentration that prevents any visible growth • For 5-fluorocytosine & Azoles, the MIC is read as the lowest drug concentration that exhibits 80% inhibition • “Trailing End Points”
- 22. Microdilution modifications • Excellent concordance between results obtained by the broth macrodilution methodology and broth microdilution adaptation • Easy to perform, trays may be sealed in plastic bags and stored frozen at -70 °C for up to six months without deterioration of drug potency • Stock solutions of antifungal agents and their intermediate concentrations are prepared in the manner described earlier • Intermediate concentrations are diluted 1:5 with RPMI in case of water soluble drug & 1:50 times for water insoluble drug to achieve 2 X (times) concentration needed for the microdilution method
- 23. ANTIMICROBIAL SOLUTION (Water Soluble Drug) (Double Strength) STE P CONC (μg/mL) SOURCE VOLUME (ml) +MEDIUM(ml) Intermediate Conc (μg/mL) Final Conc 1:5 (RPMI) (μg/mL) 1 5120 STOCK 1 ml 7 ml 640 128 2 640 Step 1 1 ml 1 ml 320 64 3 640 Step 1 1 ml 3 ml 160 32 4 160 Step 3 1 ml 1 ml 80 16 5 160 Step 3 0.5 ml 1.5 ml 40 8 6 160 Step 3 0.5 ml 3.5 ml 20 4 7 20 Step 6 1 ml 1 ml 10 2 8 20 Step 6 0.5 ml 1.5 ml 5 1 9 20 Step 6 0.5 ml 3.5 ml 2.5 0.5 10 2.5 Step 9 1 ml 1 ml 1.25 0.25 11 2.5 Step 9 0.5 ml 1.5 ml 0.625 0.12 12 2.5 Step 9 0.5 ml 3.5 ml 0.3126 0.0625
- 24. ANTIMICROBIAL SOLUTION (Water Insoluble Drug) (Double Strength) STE P CONC (μg/mL) SOURCE VOLUME (ml) + SOLVENT(ml) Intermediate Conc (μg/mL) Final Conc 1:50 (RPMI) (μg/mL) 1 1600 STOCK 1600 32 2 1600 STOCK 0.5 ml 0.5 ml 800 16 3 1600 STOCK 0.5 ml 1.5 ml 400 8 4 1600 STOCK 0.5 ml 3.5 ml 200 4 5 200 Step 4 0.5 ml 0.5 ml 100 2 6 200 Step 4 0.5 ml 1.5 ml 50 1 7 200 Step 4 0.5 ml 3.5 ml 25 0.5 8 25 Step 7 0.5 ml 0.5 ml 12.5 0.25 9 25 Step 7 0.5 ml 1.5 ml 6.25 0.125 10 25 Step 7 0.5 ml 3.5 ml 3.13 0.0625
- 25. Continued. . . • A stock yeast suspension of 0.5 Mc Farland is prepared • 0.5 McF corresponds to 1 x 106 to 5 x 106 • Suspension is then diluted 1:50 (2 x 104 to 5 x 106) • Further diluted 1:20 with RPMI to achieve the 2 x test inoculum (5 x 102 to 2.5 x 103) = (1 x 103 to 5 x 103) CFU/ml) • When combined with drug, the final concentration will be 5 x 102 to 2.5 x 103 CFU/ml
- 26. Continued . . . • Using a multichannel pipette, dispense 100 ꭒl of the 2x antifungal concentrations into columns 1 to 10 of sterile- disposal 96-well (U-shaped) microdilution plates • Column 1 will contain the highest concentration, and column 10 will contain the lowest concentration of drug • Columns 11 and 12 should each receive 100 ꭒl of diluent • Using a multichannel pipette, dispense 100 ꭒl of working yeast suspension into each well of columns 1 to 11 • Column 11 will serve as Growth Control • Column 12 should remain uninoculated & is used as a sterility control
- 28. Continued. . . • Tubes should be incubated without agitation at 35°C for 46 to 50 hr (Candida spp.) or 70 to 74 (C. neoformans) hr • Plates are placed on a microdilution plate reader with a magnifying mirror • MICs are read in the same manner as described for macrodilution tubes
- 29. Testing of Filamentous Fungi • As per CLSI M38/A2 • Test medium of choice for filamentous moulds is RPMI 1640 • Preparation of both stock and working solutions of antifungal agents remains the same as described • Allylamine terbinafine can be added to the list of antifungal agents, in particular when testing dermatophytes • Terbinafine is dissolved in DMSO and tested at final conc. of – 16 to 0.03 ꭒg/ml for filamentous moulds 0.5 to 0.001 ꭒg/ml for dermatophytes
- 30. Continued. . . • Aspergillus spp., Pseudallescheria boydii, Rhizopus, and Sporothrix - Grown on PDA slants at 35°C for 7 days • Fusarium spp Grown on PDA slants at 35°C for 2 to 3 days and then at room temperature until day 7 • Trichophyton rubrum isolates can be induced to form conidia on cereal agar plates incubated at 30°C for 5 to 7 days
- 31. Continued. . . • Slants are covered with sterile saline and conidia are harvested by agitation with a Pasteur pipette • One drop of Tween 20 is added to facilitate inoculum formation • Resulting mixture of conidia & hyphal particles is removed to a sterile conical tube and allowed to settle for 3 to 5 min • Upper layer is removed to a second vial and vortexed • Optical density (OD) of this suspension is measured using Densitometer and adjusted with sterile saline - Aspergillus spp. and Sporothrix, 0.09 to 0.11 OD units Fusarium, P. boydii, and Rhizopus, 0.15 to 0.17 OD units
- 32. Continued. . . • Stock suspensions of conidia is to be diluted 1:50 in RPMI 1640 for microdilution testing • Final test inocula suspension should be in the range of 0.4 x 104 to 5 x 104 • Procedures for setting up the macro- and microdilution tests are the same as described above for yeast isolates
- 33. Reading Result • For conventional microdilution procedure, the growth in each MIC well is compared with that of the growth control with the aid of reading mirror • Each microdilution well is then given a numerical score as follows : 4 – No reduction in growth 3 – Approximately 75% reduction in growth 2 – Approximately 50% reduction in growth 1 - Approximately 50% reduction in growth 0 – Optically clear or no growth
- 34. Continued. . . • For AMB – End point well defined MIC is read as the lowest concentration that prevents any discernible growth (Score 0) • Flucytosine, Fluconazole & Ketoconazole End point typically less defined; Less stringent end point is allowed Turbidity that corresponds to approximately 50% reduction in growth compared to the growth control well (Score 2)
- 35. Continued. . . • Itraconazole & newer triazoles - Posaconazole, Ravuconazole & Voriconazole End point well defined (Score 0) – For Aspergillus spp When testing for Dermatophyte isolate, 80% or more reduction in turbidity relative to the growth control • Terbinafine Turbidity allowed is corresponds to 80% or more reduction in growth relative to growth control • Recommended MIC / MEC limits for Quality Control & Reference strains are given in CLSI M27/A3 & M38/A2 document
- 36. CALORIMETRIC METHOD • Based on microdilution format as CLSI • Reading end points are enhanced by the use of Calorimetric indicator (Modified Resazurin) • Blue – No growth • Red – Growth • Purple – Partial inhibition
- 37. ANTIFUNGAL DISK DIFFUSION SUSCEPTIBILITY TESTING (CLSI M44/A2) • Method described is intended for testing Candida spp • Does not encompass any other genera & has not been used in the studies of the yeast form of the Dimorphic fungi REAGENTS FOR THE DISK DIFFUSION TEST • Mueller-Hinton Agar + 2% Glucose + 0.5 mcg/ml Methylene Blue Dye medium (GMB); pH 7.2 to 7.4 • 2% glucose provides for suitable fungal growth • Methylene blue enhances zone edge definition
- 38. Continued. . . Preparation of test inoculum Inoculation of the test plate (Same as that for bacteria) Application of Disks to inoculated agar plate
- 39. Reading plates & interpreting results • Examine each plate after 24 to 24 Hr of incubation • Measure the zone diameter to the nearest whole millimeter at the point at which there is a prominent reduction in growth • Pinpoint microcolonies at the zone edge or larger colonies within a zone are encountered frequently & should be ignored
- 40. Continued. . . INTERPRETIVE CATEGORIES • Susceptible (S) : Infection due to the strain may be appropriately treated with the dose of the antimicrobial agent recommended • Susceptible Dose Dependent (S-DD) : includes isolates with antimicrobial agent MICs that approach usually attainable blood & tissue levels but the response rate may be lower than for susceptible isolates (Only applies when multiple approved dosage options exist) • Resitant (R) : Isolates are not inhibited by the usually achievable concs of the agent with normal dosage schedules
- 41. ANTIFUNGAL AGENTS, ZONE DIAMETER & MIC INTERPRETIVE CRITERIA FOR C. albicans & C. tropicalis Antimicr obial agent Dics Diffusion Zone Diameter (mm) MIC (mcg/ml) Suscep -tible Interme- diate Resistant Suscept- ible S-DD Interm- ediate Resistant Anidul- afungin - - - ≤ 0.25 - 0.5 ≥ 1 Caspof- ungin ≥17 15-16 ≤14 ≤ 0.25 - 0.5 ≥ 1 Micafu- ngin ≥22 20-21 ≤19 ≤ 0.25 - 0.5 ≥ 1 Flucon- azole ≥17 14-16 ≤13 ≤ 2 4 - ≥ 8 Itracon- azole ≤ 0.125 0.25-0.5 - ≥ 1 Voricon- azole ≥17 15-16 ≤14 ≤ 0.125 0.25-0.5 - ≥ 1
- 42. Antifungal Agents Disk Content (mcg) Recommended Quality Control Zone Diameter (mm) Ranges C. albicans C. parapsilosis C. tropicalis Fluconazole 25 mcg 28 -39 22 – 33 26 - 37 Voriconazole 1 mcg 31 – 42 28 – 37 * Quality control ranges have not been established for these strains / antimicrobial agent combination due to extensive inter-laboratory variation
- 43. E–TEST (EPSILOMETER TEST) • A predefined stable antimicrobial gradient is present on a thin inert non-porous plastic carrier strip 5mm wide, 60 mm long known as E-test strip • Device consists of a predefined, continuous, and exponential gradient of antibiotic concentrations immobilized along a rectangular plastic test strip • Following incubation, a symmetrical inhibition ellipse is produced • Intersection of the inhibitory zone edge and the calibrated carrier strip indicates the MIC value
- 44. Continued. . . • Following incubation, a symmetrical inhibition ellipse is produced • Intersection of the inhibitory zone edge and the calibrated carrier strip indicates the MIC value
- 46. Continued. . . Microorganism YEAST MOULD Medium RPMI 1640 + 2% glucose + MOPS + 1.5% Bacto agar RPMI 1640 + 2% glucose + MOPS + 1.5% Bacto agar Inoculum 0.5 McF (for Candida spp) (1McF for Cryptococcus neoformans); Suspension made in Saline 0.5 McF Aspergillus spp. (1 for Fusarium, Rhizopus spp); Suspension is made in Saline + Tween 20 Time/ Temp 24 Hrs at 35°C for Candida spp 16 to 72 Hrs depending on genus at 35°C MIC Panel FL, IT, VO, AMB, CS, FC AMB, IT, CS, POS, VO
- 47. VITEK 2 COMPACT
- 48. VITEK 2 COMPACT Suspension Preparation • Transfer a sufficient number of colonies of a pure culture in 3.0 mL of sterile saline (aqueous 0.45% to 0.50% NaCl, pH 4.5 to 7.0) in a 12 x 75 mm clear plastic (polystyrene) test tube • Turbidity is adjusted accordingly & measured using DensiChek • For Yeast & Yeast like organism turbidity should be between 1.8 – 2.2 McF • In a second tube containing 3.0 mL of saline, transfer 280 μL of the the suspension prepared earlier & the AST card is put on the corresponding slot
- 49. Continued. . . • Reagent cards have 64 wells that can each contain an individual test substrate • Each AST card contains 64 microwells • Control well contains only the microbial culture media, with the remaining wells containing premeasured amounts of specific antimicrobials combined with culture medium • Card is then filled, sealed, and thereafter placed into the instrument incubator/reader manually / automatically • Each card is removed from the carousel incubator once every 15 minutes • Transported to the optical system for reaction readings, and then returned to the incubator until the next read time
- 50. Continued. . . • Data are collected at 15-minute intervals during the entire incubation period • Test data from an unknown organism are compared to the respective database to determine a quantitative value for proximity to each of the database taxa • Each of the composite values is compared to the others to determine if the data are sufficiently unique or close to one or more of the other database taxa • If a unique identification pattern is not recognized, a list of possible organisms is given, or the strain is determined to be outside the scope of the database
- 51. Continued. . . AST CARD • Instrument monitors the growth of each well in the card over a defined period of time upto 36 Hrs for Yeast • At the completion of the incubation cycle, MIC values are determined for each antimicrobial contained on the card automatically by the VITEK machine
- 53. CONCLUSION • While promising, antifungal susceptibility testing is still a research tool • In view of the AIDS epidemic & increasing incidence of invasive mycosis and life threatening infections there is a need to institute antifungal susceptibility in all tertiary care hospitals • Helps to evaluate therapeutic outcome • Surveillance required to assess the in vitro activity of newer antifungal drugs & know the emerging resistance pattern
- 54. THANK YOU
Editor's Notes
- #3: It was only with the development of 5-fluorocytosine and, more recently, the azole antifungal agents that differences within and between species started to become apparent. FALLOW – Ploughed & left unseeded for a season or more; uncultivated
- #4: has been attributed to such factors as the increasing use of cytotoxic and immunosuppressive drugs to treat both malignant and nonmalignant diseases, the increasing prevalence of infection due to human immunodeficiency virus type 1, and the widespread use of newer and more powerful antibacterial agents
- #6: EIEIO – Emerging infection & epidemiology of Iowa oraganism. SCOPE – Surviellance & control of pathogen of Epidemiological importance Quebec – a city in CANADA which sits at St Lawrence river
- #7: From 1999 to 2006, Candida isolates were differentiated between C. albicans & NAC spp. Between 2006 and 2008, Candida isolates was speciated to the species level & antifungal susceptibility test was done. C. tropicalis was the most common species (182 cases; 29.2%), followed by C. albicans (105 cases; 16.8%) and C. haemulonii (97 cases; 15.5%). C. parapsilosis and C. glabrata were isolated in 78 (12.5%) and 53 (8.5%) cases, respectively. These five species constituted 82.6 per cent of the isolates. C. krusei was isolated in only 11 cases, C. pelliculosa 23 cases, Pichia ohmeri 10 cases, C. rugosa 9 cases and Trichosporon spp. 7 cases
- #8: Terbinafine not as much effective as itraconazole for non dermatophytic infection…Also effective aspergillosis,chromoblasto,sporotrich….but not licensed yet
- #9: Microtubules – are conveyer belts inside the cells. They move vescles, granules, organelles like mitochondria & chromosomes via special attachment proteins. They serve a cytoskeletal role too. AMB is the DOC for Cryptococcal meningitis where as cryptococcus else where DOC is Ketoconazole Terbinafine not as much effective as itraconazole for non dermatophytic infection…Also effective aspergillosis,chromoblasto,sporotrich….but not licensed yet. Lipid Amp B reduce renal toxicity
- #10: Terbinafine not as much effective as itraconazole for non dermatophytic infection…Also effective aspergillosis,chromoblasto,sporotrich….but not licensed yet Caspofungin is approved only for invasive aspergillosis not responding to AMB or Voriconazole. It is non toxic & cause only infusion related reaction Lipid Amp B reduce renal toxicity
- #12: RPMI (Roswell park memorial institute) is a broth medium without sodium bicarbonate which is supplemented with L-glutamine and a pH indicator 10.4 g powdered RPMI 1640 medium (with glutamine and phenol red, without bicarbonate) 34.53 g MOPS (3-[N-morpholino] propanesulfonic acid) buffer Dissolve powdered medium in 900 mL distilled H2O. Add MOPS (final concentration of 0.165 mol/L) and stir until dissolved. While stirring, adjust the pH to 7.0 at 25 oC using 1 mol/L sodium hydroxide. Add additional water to bring medium to a final volume of 1 L. Filter sterilize and store at 4 °C until use
- #13: Stock solutions of antifungal agents Do not commonly support the growth of contaminating organisms and can be considered sterile
- #14: Acceptable powders bear a label that states the drug generic name, its potency (ꭒg/mg) & its expiration date
- #16: Small volume of the sterile stock solution are dispensed into sterile polypropylene or polyethylene vials, carefully sealed & stored.
- #17: Breakpoint concentration is a chosen concentration (mcg/ml) of an antimicrobial agent which defines whether a species of bacteria or fungus is susceptible or resistant to the antibiotic. If MIC is less than or equal to the susceptibility breakpoint the organism is considered sensitive to the particular antimicrobial agent. If the MIC is greater than this value, the organism is considered intermediate or resistant to the antibiotic.
- #20: 24 (Candida spp.) or 48 Hr (C. neoformans) . 0.5 McFarland standard (spectrophotometer reading at a k of 530 nm) yield a concentration of 1 x 106 to 5 x 106 cells per m
- #21: (at the intermediate concentration for water-soluble drugs and the intermediate concentration diluted 1:10 for water-insoluble drugs Tubes should be incubated without agitation at 35C for 46 to 50 (Candida spp.) or 70 to 74 (C. neoformans) h
- #22: The azoles will often show “trailing endpoints,” where turbidity persists in all drug concentrations above the MIC. To reduce variability in reading these endpoints, the amount of allowable turbidity can be demonstrated by diluting 0.2 ml of the growth control in 0.8 ml of RPMI For amphotericin B, end points are typically well defined, and the MIC is easily read as the lowest drug concentration that prevents any discernible growth. For flucytosine and especially for azoles such as fluconazole and ketoconazole, end points are typically less well defined than that described for amphotericin B which may contribute to a significant source of variability. Application of a less stringent end point (allowing some turbidity above the MIC) has improved interlaboratory agreement and also discriminates between putatively susceptible and resistant isolates. When turbidity persists, it is often identical for all drug concentrations above the MIC. The amount of allowable turbidity can be estimated by diluting 0.2 mL of drug-free control growth with 0.8 mL of media, producing an 80% inhibition standard
- #26: Final concentration of yeast suspension in macrodilution procedure is 5 x 102 to 2.5 x 103. In microdilution procedure – 2 x (5 x 102 to 2.5 x 103)
- #31: Certain dermatophyte isolates pose special problems, as conidiation is often absent on potato dextrose agar. Trichophyton rubrum
- #38: MHA (Conventional) – Beef extract, Casein Hydrolysate, Starch, Agar, d.w. No Glucose & Methylene blue All organism needs to be subcultured on SDA to ensure purity. Inoculum is prepared by picking 5 colonies of 1mm diameter from a 24 hr old culture of Candida species. Colonies are emulsified in 5 ml of sterile 0.85% saline. Resulting suspension is vortexed for 15 sces & 0.5 Mc Farland turbidity adjusted either visually or with a spectrophotometer.
- #39: The disk should be distributed evenly over the agar surface so that they are not closer than 24 mm from center to center. Ideally not more than 12 disks should be placed on a 150mm plate or more than 5 disks on a 100 mm plate. Because the drug diffuses almost instantaneously, no disks should be moved once placed at a particular site. If need be a new disk is to be placed at a new location. The plate are inverted & incubated at 35 C +/- 2C within 15 mins aafter the disks are applied. 0.5 Mc Farland standard allows transmittance of light at 530 nm.
- #40: How to read the plate ? The pate is held a few inches above a black, nonreflecting background illuminated with reflected light.
- #41: Susceptible (S) : Infection due to the strain may be appropriately treated with the dose of the antimicrobial agent recommended for that type of infection & infecting species unkess otherwise contraindicated. S-SD : Susceptibility is dependent on reaching maximal possible blood level. This category also includes a buffer zone, which should prevent small, uncontrolled, technical factors from causing major discrepencies in interpretations, esp fr drugs with narrow pharmaco toxicity margins Intermediate : Available data does not clearly permit them to categorise as either susceptible or resistant
- #42: Intermediate: Implies clinical efficacy in body sites where drugs are physiologically concentrated (eg. Quinolones & beta lactams in urine or higher than normal dosage of the drug can be used (eg. Beta lactam). Provides Buffer Zone. S-SD : Only applies when mutiple approved dosage options exist. Same clinical response as ‘S’ is expected if higher or frequent dosing is used.
- #44: Principle : quantitative method that applies both the dilution of antibiotic and diffusion of antibiotic into the medium When applied onto an inoculated agar plate, there is an immediate release of the drug.
- #47: 48 to 72 Hrs at 35°C for C. neoformans
- #49: Pre-requisite for the test is to perform a gram stain 1. GN - Gram-negative fermenting and non-fermenting bacilli (0.5-0.63) 2. GP - Gram-positive cocci and non-spore-forming bacilli (0.5-0.63) 3. YST - yeasts and yeast-like organisms (1.8-2.2) 4. BCL - Gram-positive spore-forming bacilli (1.8-2.2)
- #50: Accommodate the same colorimetric reagent cards that are incubated and interpreted automatically
- #52: The AST card is essentially a miniaturized and abbreviate version of the doubling dilution technique for MICs determined by the microdilution method