Ca esophagus 12th
- 1. CARCINOMA ESOPHAGUS Presentor : Dr.Gowtham Moderator: Dr.Vikas Choudhary
- 2. Epidemiology and incidence • Esophageal cancer is the eighth most common cancer worldwide • Incidence of 160/100,000 in parts of South Africa and China and 540/100,000 in Kazakhstan. • India 8-20 / 100,000 , 6th most common in males • Squamous cell carcinoma still accounts for most esophageal cancers diagnosed. • M:F – 3:1 (SCC) .. …..15:1 (adeno) • Adeno – whites …..SCC – african American Globocan 2018
- 3. Anatomy • The esophagus is a thin-walled, hollow tube approximately 25 cm in length. • It is lined with stratified keratinized squamous epithelium, extending from the cricopharyngeus muscle at the level of the cricoid cartilage superiorly to the gastroesophageal junction inferiorly. • The esophageal wall is composed of three layers: the mucosa, submucosa, and muscularis propria • The mucosal layer contains the epithelium(M1), lamina propria(M2), and muscularis mucosae(M3). • The epithelium is separated from the lamina propria by a basement membrane. In the portion of the esophagus containing columnar-type epithelium, the muscularis mucosae may consist of two layers. • Similarly, the submucosal layer may be divided into inner (SM1), middle (SM2), and outer(SM3) layers. The muscularis propria consists of a circular inner layer and longitudinal outer layer. • The adventitia (periesophageal connective tissue) lies directly on the muscularis propria. • No serosa is present, facilitating extraesophageal spread of disease. • AJCC 8th 4 Parts : cervical , upper thoracic ,middle thoracic ,lower thoracic
- 4. Siewert’s classification • Type I : From >1 cm up to 5 cm above the gastroesophageal junction (Z line), the tumor is classified as a carcinoma of the distal esophagus • Type II : Within 1 cm cephalad to 2 cm caudad to the gastroesophageal junction. • Type III :Tumor center is located >2 cm below the gastroesophageal junction AJCC 8th • Cancers with an epicenter in the lower thoracic esophagus or gastroesophageal junction or within the proximal 2 cm of the stomach (i.e.,cardia) and extending up to the GE junction or esophagus are staged as a carcinoma of the esophagus. • If the epicenter is >2 cm distal to the gastroesophageal junction, these are classified as stomach cancers.
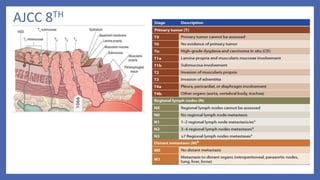
- 5. Staging • AJCC(TNM) • Tumor • Lymph node(N0,N1) • Metastasis • Most widely accepted ELLIS (WNM) • Wall penetration • Lymph node(N0,N1,N2) • Metastasis
- 6. AJCC 8TH
- 7. AJCC -8th SCC • Grade ,location of tumour affect outcomes Adenocarcinoma • Tumour location –not a prognostic variable Adenosquamous – staged as squamous only
- 8. p Stage yp Stage (post neoadjuvant)
- 9. Lymphatics • The esophagus has an extensive, longitudinal interconnecting system of lymphatics. The esophageal lymphatic network is primarily located within the submucosa .However, channels are also present within the lamina propria, facilitating spread of even superficial cancers of the esophagus involving the mucosa. • In addition to these longitudinal lymphatics, intramural lymphatics may traverse the muscularis propria, facilitating tumor spread to regional lymphatic channels and paraesophageal nodes. • Lymph can travel the entire length of the esophagus before draining into lymph nodes, the entire esophagus is at potential risk for lymphatic involvement. • Up to 8 cm or more of “normal” tissue can exist between gross tumor and micrometastases “skip areas”. • Lymphatics of the esophagus drain into nodes that usually follow arteries,including the inferior thyroid artery, the bronchial and esophageal arteries, and the left gastric artery (celiac axis).
- 10. LN Status • Depth of tumor penetration (T stage) affects lymph node involvement (LNI) • Intramucosal T1 lesions (18% LNI) • submucosal T1 lesions (55% LNI) • T2 lesions (60% LNI) • T3 lesions (80% LNI) • Chance if LN <50% - conservative eso resection and limited lymph node dissection • LN>50% - neoadjuvant therapy followed by resection
- 11. Natural history and pattern of spread • Squamous cell carcinoma is characterized by extensive local growth and proclivity to lymph node metastases. • Because the esophagus has no covering serosa, direct invasion of contiguous structures may occur early. • Lesions in the upper esophagus can impinge on or invade the recurrent laryngeal nerves,carotid arteries, and trachea. If extraesophageal extension occurs in the mediastinum, tracheoesophageal or bronchoesophageal fistula may occur. • Tumors in the lower one-third of the esophagus can invade the aorta or pericardium, resulting in mediastinitis, massive hemorrhage, or empyema.
- 12. Histology • Squamous and adeno -95% of all carcinomas • Psuedosarcoma – spindle cell carcinoma (SCC variant) • Adenoca variants –Adenoid cystic ,mucoepidermoid poorer prognosis • Small cell carcinoma – arise from argyrophilic cells paraneoplastic syndromes !! (ADH,hypercalcemia) , similar to SCLC • Nonepithelial origin – very rare • Malignant melanoms –MS 7months • Lymphoma –mostly extension , as primary very rare
- 13. SCC • Squamous cell carcinomas arise from the squamous mucosa - native to the esophagus - 70% - upper and middle thirds • Most common type of esophageal ca in India (90%) • Smoking and alcohol are common eitiologic factors (5 fold increase in risk) • Combined increase risk from 25 - 100 folds • Dietary • Nitrosamines (pickled foods , smoked food) • long term ingestion of hot liquids • Micronutrient deficiency (Vit. A, B12, C, E). • Trace Element deficiency (Cobalt, Copper & Selenium) • Acquired • Cigarette smoking • Alcohol. • Chronic esophagitis. • Chronic Dysphagia • Caustic ingestion • Radiation exposure • Plummer – vinson syndrome • Tylosis(40%) • Achalasia (16 -fold) • Esophageal strictures and diverticula • p53 • Premalignant conditions
- 14. Adenocarcinoma • almost 70 % - United States and Western countries. Etiology : • Increasing incidence of GERD • Western diet • Increased use of acid-suppression medications Histologically it is from : • Submucosal glands of the esophagus • Heterotopic islands of columnar epithelium • Malignant degeneration of metaplastic columnar epithelium (Barrett’s esophagus) – 40 fold increased risk
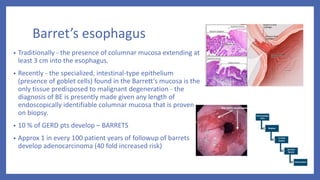
- 15. Barret’s esophagus • Traditionally - the presence of columnar mucosa extending at least 3 cm into the esophagus. • Recently - the specialized, intestinal-type epithelium (presence of goblet cells) found in the Barrett’s mucosa is the only tissue predisposed to malignant degeneration - the diagnosis of BE is presently made given any length of endoscopically identifiable columnar mucosa that is proven on biopsy. • 10 % of GERD pts develop – BARRETS • Approx 1 in every 100 patient years of followup of barrets develop adenocarcinoma (40 fold increased risk)
- 16. Clinical manifestations • Early - asymptomatic – mimic GERD (3-4 months before diagnosis) • Dysphagia >90% (mc) > 2/3rds of lumen has to be obstructed (lack of serosa) • Weight Loss (40-70%) -most common symptoms • Vomiting/Regurgitation • Pain. • Cough , choking , asp.pneumonia (TEF) • Hoarseness.(left.RLN , vocal cords) • Dyspnoea • In high-incidence areas where screening is practice,the most prominent early symptom is pain on swallowing rough or dry food • Systemic disease – jaundice ,excessive pain ,bone pain,respiratory symptoms Perez & Brady 7E
- 17. Investigations • Endoscopy • CT • PET • MRI • EUS
- 18. Esophagoscopy : • Good 1st test – dysphagia & suspecting ca esophagus • Can differentiate intra luminal from intramural & intrinsic from extrinsic • Dx of esophageal ca is best made by endoscopic biopsy Critical points : • Location of lesion • Nature of lesion (polypoid etc) • Extent & relationship to cricophayngeus ,GEJ Apple core appearance
- 19. : CT • Imp for staging. • Chest and abdomen – Length , thickness, LN Liver and lung mets , T4 • Accuracy 57%- T 74%- N , 83%- M • Many unresectable tumors by CT scan are deemed resectable at the time of surgery.
- 20. PET : • FDG –PET: • Evaluates Primary mass LN ,Mets • Sensitivity and specificity slightly greater than CT • Not reliable as single Dx tool • Value in evaluating response to chemo and RT • N staging imitations :periesophageal ln difficult to distinguish from primary mass , infections • Detect distant metastasis in 20% pts • (which was Not detected on CT ) • PET + CT – improve accuracy of detecting • -stage III by 23% • -stage IV by 18% Figure Distant lymph node metastases of esophageal cancer detected by integrated CT PET. A, Integrated CT PET demonstrates para-aortic lymph node metastases showing increased FDG uptake (arrowheads). B, Corresponding CT image shows lymph nodes (arrowheads) measuring 5 to 8 mm in diameter. Based on size criteria, these lymph nodes may be considered benign on CT scan
- 21. MRI • Not done routinely • To identify involvement of vascular & neural • Accurately detects T4 and mets • But overstages T & N status
- 22. EUS(Endoscopic Ultrasonography) • More accurately assess -periesophageal & celiac LN involvement -transmural extent of ds. • Limitation : -less significant accuracy following neoadjuvant therapy d/t not discriminate tumor from postradiation inflammation & fibrosis • Overall accuracy for Local nodal (N) staging 75% to 80% • Overall accuracy for Tumor staging (T) 85% to 90%
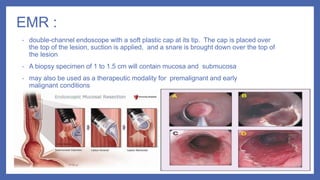
- 24. EMR : • double-channel endoscope with a soft plastic cap at its tip. The cap is placed over the top of the lesion, suction is applied, and a snare is brought down over the top of the lesion • A biopsy specimen of 1 to 1.5 cm will contain mucosa and submucosa • may also be used as a therapeutic modality for premalignant and early malignant conditions
- 25. Minimal invasive surgical modalities for staging: • Includes bronchoscopy ,Thoracoscopy and Laparoscopy. • Highly accurate in evaluating N & M Status.
- 26. Molecular considerations • HER2/neu oncogene : overexpressed in 15% to 30% of adenocarcinomas and 5% to 13% of SCC , Both IHC and FISH to be done , associated with tumor invasion, lymph node metastases, and poor prognosis. • EGFR :Overexpression of EGFR on IHC testing occurs in approximately 80% of patients with adenocarcinoma and SCC , research for targeted therapies ongoing, poor prognosis in terms of decreased survival. • (VEGF) expression levels: poor prognosis , targeted therapy (ramucirumab) • COX-2:Inhibition of COX-2 activity results in enhanced radiosensitization of tumor tissue but not normal tissue.
- 28. Treatment options • Surgery • Radiation therapy 1.External Radiotherapy 2.Intraluminal Brachytherapy • Chemotherapy • Multimodality treatment combining above methods
- 29. Scheme of management Perez 7th edition
- 30. SURGERY RESECTABLE ESOPHAGEAL CANCERS: (STAGE I –III) UNRESECTABLE ESOPHAGEAL CANCERS:
- 31. Endoscopic methods • Endoscopic Mucosal Resection- • CONSIDERED as essential diagnostic, staging and therapeutic option for patient having high grade dysplasia, superficial esophageal tumor • Procedure – injection of fluid in submucosal layer or use the suction to trap the lesion into cylinder followed by tissue retrieval by snare or endoscopic knife • Complete local remission rate – 99-100% • 5 yr survival rate – 98%
- 33. CERVICAL ESOPHAGUS Proximal esophageal tumors are treated with DEFINITIVE CHEMORADIOTHERAPY Total esophagectomy with resection of pharynx, larynx, thyroid gland is required (Total pharyngo-laryngo-esophagectomy) – high morbidity with loss of function.
- 34. THORACIC ESOPHAGUS IVOR LEWIS OPERATION Abdomen opened first and stomach mobilized And then Right thoracotomy -Oesophagogastric anastomosis 2 stage approach McKeown OPERATION 3 incisions Abdominal, right posterolateral thoracotomy and left cervical incision
- 35. TRANSHIATAL ESOPHAGECTOMY without thoracotomy ( ORRINGER’S OPERATION ) Avoidance of thoracotomy incision Avoidance of mediastinitis Shorter Duration of operation Poor visualization of upper and middle esophageal tumors Anastomotic leak Chylothorax Recurrent laryngeal nerve injury DisadvantagesAdvantages
- 36. Lymph node dissection • En Bloc resection is the best treatment with removal of all lymph nodesEn bloc esophagectomy involves resection of middle and lower esophageal tumors with an envelope of adjacent tissue that includes the mediastinal pleura laterally, the pericardium anteriorly, and the azygos vein and thoracic duct posterolaterally with the surrounding periesophageal tissue and lymph nodes
- 37. Transhiatal vs transthoracic approach Transhiatal approach Transthoracic approach Thoracotomy not required Required Distal esophagus Middle or distal esophagus Less pulmonary complications more Anastmotic leak high low Disease free survival 1.4yr 1.7yr Overall survival 1.8yr 2.0yr 5 yr survival rate 34% 36% In hospital mortality 2% 4% • Conclusion- either the transhiatal or transthoracic procedure can be performed with acceptable mortality and morbidity , with either technique the outcome is almost similar
- 38. Surgery Alone as Treatment • 5 yr survival rate 28% , compared to 10% in patients treated medically. • Locoregional failure rate 32-45% • Need for adjuvant therapy • Conclusion- as relapse rate is high and long term survival is poor , integration of adjuvant or neoadjuvant chemoradiation approaches in treatment is rational and indicated. • Optimum time for surgery ? (After NACRT) Traditional 4-6 weeks Vs >12 weeks Perez and Brady’s 7th edition
- 39. Radiation Pre op RT Pre op CT RT Post op RT Radical RT Radical CT RT Palliative Brachytherapy
- 40. Role of Preoperative Radiation Therapy • In various trials comparing surgery alone vs preoperative radiation followed by surgery it was found that there is no clinical benefit of using preoperative radiation. • No significant survival benefit • Local control rate was improved but non significant. • Conclusion- preoperative radiation therapy is not recommended • Dosage - 41.4-50.4 Gy/ 1.8-2.0 Gy per fraction/ 23-28 Fr
- 41. Role of Preoperative Chemoradiation • It is most common approach for resectable esophageal cancer • Benefits downstaging of disease increase rate of complete resection of disease with negative margins eradicate occult micrometastatic disease • Improves local control • Absolute Survival benefit 2yr - 13% 5yr - 6.5%
- 43. Median Survival was 49 months as compared to 24 months in surgical arm – DOUBLED !!
- 44. Regimens • Paclitaxel+carboplatin (cat-1) Paclitaxel 50mg/m2 I V &+ Carboplatin AUC 2 Day-1 Wkly x 5 wk • Cisplatin+ Fluorouracil (cat-1) Cisplatin 75-100 mg /m2 D1&D29 + Fluorouracil 750-1000 mg /m2 IV infusion over 24 hours daily on D1- 4 & D 29–32 {35day cycle} OR Cisplatin 15 mg /m2 D1 - 5 + Flourouracil 800 mg /m2 IV infusion over 24 hours daily on D1-5- 21days cycle x 2 • Fluorouracil+Oxaliplatin Oxaliplatin 85 mg /m2 IV on D1 + Leucovorin 400 mg /m2 D1+ FU 400 mg /m2 IV Push D1 and 800 mg /m2 IV continuous infusion over 24 hrs daily on D1 & D2 14 days cycle x 3 with radiation and 3 after radiation
- 45. Radiotherapy Alone as Treatment • Indications- inoperable disease - medical contraindication of surgery - as palliative treatment • Median survival – 6-12 month • 5 yr survival - <10% (I- 20%, II- 10%, III- 3%, IV- 0%) • When compared to chemoradiation the 3 yr survival rate in radiation therapy alone group was found 0% • Conclusion- treatment with radiation therapy alone for esophageal cancer is palliative in majority of patients and for radical treatment it should be used in conjunction with other modalities.
- 46. Definitive Chemoradiation Indications :- • In squamous cell carcinoma -cT1b-cT4a, N0-N+ - Cervical esophagus - If patient refuses surgery - cT4b • In adenocarcinoma esophagus - cT1b-cT4a, N0-N+ - If patient refuses surgery - cT4b
- 47. Definitive Chemoradiation • In comparison to radiation therapy alone – Improved median survival 14 vs 9 months Improved 5 yr survival rate 27 % vs none • When compared to surgery alone in a resectable esophageal tumor no difference in survival rate, local failure, treatment related mortality. • 5 yr survival rate – 27% • Radiation Dose – 50-50.4 Gy/ 1.8-2.0 Gy per fraction/25-28 Fr
- 48. CHEMOTHERAPY REGIMENS USED IN DEFINITIVE CHEMORADIATION • Cisplatin+ Fluorouracil 5-FU (cat-1) ( Cisplatin 75-100 mg /m2 D1 + 5-FU 750-1000 mg /m2 IV infusion over 24 hours daily on D1- 4 {28 day cycle} x 4) 2 cycles with RT F/b 2 cycles without RT • Oxaliplatin + 5FU (cat-1) Oxaliplatin 85 mg/m2 D1+ Leucovorin 400 mg/m2 IV D1 + 5-FU 400 mg/m2 IV D1 + 5-FU 800 mg/m2 IV over 24 hours D1 & D2 2weekly - 3cycles with RT f/b 3cycles without RT OR Oxaliplatin 85 mg/m2 IV on D1,D15,D29 for 3 doses Fluorouracil 180 mg/m2 IV Daily on D1-33
- 49. Post operative radiation therapy • No survival advantage • May decrease time to local recurrence particularly in cases with involved margins • no significant decrease in local or distal failure rates • Dosage - 45-50.4 Gy/ 1.8-2.0 Gy per fraction/ 25-28 Fr Post operative Chemoradiation (in case of R1 ,Node positive(adeno carcinoma), pT3 ,pT4a) • When compared to surgery alone, surgery followed by chemoradiation group showed • 5FU +Leucovorin based chemotherapy • Significant decrease in local failure – 19% vs 29% • Significant survival advantage – 27 vs 36 months • Long term followup (>10yr) – continued to show survival advantage • Conclusion- it is appropriate to advise adjuvant chemoradiation in view of improved local control and survival
- 50. RADIOTHERAPY TECHNIQUES PATIENT POSITIONING & IMMOBILISATION : • Cervical /upper third : Supine with arms by the side with straight cervical spine & parallel to couch top + Immobilisation of jaw ,neck & upper thorax • Middle and lower third: Supine with arms above their head + Vertebral column should be as parallel to couch as possible GEJ tumours and involving stomach fasting 2 hours before simulation for reproducibility.
- 51. Upper Esophagus Radiation Field • .Superior: 5 cm proximal to tumor + supraclavicular LN + upper mediastinal LN Inferior: 5 cm distal to tumor Lateral: Tumor + 2.5 –3 cm + mediastinal LN + 2/3 of clavicle for SCF LN
- 52. Middle esophagus Radiation field Superior: 5 cm proximal to tumor + upper mediastinal LN Inferior: 5 cm distal to tumor + mediastinal LN Lateral: tumor + 2.5–3 cm + mediastinal LN
- 53. Lower esophagus RT Superior: 5 cm proximal to tumor + mediastinal LN Inferior: 5 cm distal to tumor + mediastinal LN + celiac LN (until L1–2 vertebrae) Lateral: tumor + 2.5–3 cm + mediastinal LN
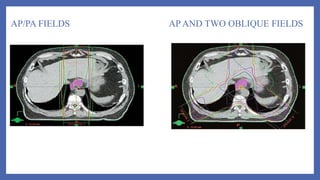
- 54. Fields commonly used:- • AP/PA fields followed by AP/Right posterior oblique(RPO)/Left posterior oblique(LPO) with or without boost • AP/PA approach followed by Right anterior oblique(RAO)/LPO fields with or without boost • 3-field technique (AP/PA with left lateral or oblique field) • 45 Gy by AP/PA field followed by additional 5.4 Gy using oblique or lateral fields –mc used.
- 55. AP/PA FIELDS AP AND TWO OBLIQUE FIELDS
- 56. Nodal basins to be covered under CTV:- • Cervical & Upper Esophagus – lower cervical and supraclavicular to subcarinal lymph nodes + upper paraesophageal lymph nodes • Middle Esophagus – Complete coverage of paraesophageal lymph nodes ( individualized field design) • Lower Esophagus – Subcarinal to left gastric and common hepatic artery/celiac lymph node inferiorly • Adenocarcinoma – similar to lower thoracic esophagus but paraesophageal lymph nodes also to be included • Middle and lower paraesophageal lymph nodes should be included in Type 1 ,Type 2 tumor with T2-T4 • Splenic and common hepatic artery nodes can be spared in Type 1 T2 tumor • GTV– primary gross tumor + nodal gross disease based on diagnostic studies • CTV– to cover subclinical disease 3-5 cm proximal and distal margins covers 94-100% subclinical disease. 2 cm radial margin • PTV– CTV + 1cm radial, 1.5cm distal , 1cm proximal margin (as per departmental protocols) IMRT NCCN 2019
- 57. External Radiation doses and OAR constraints • Higher doses 60-66 Gy in cervical esophagus , but there is little evidence beyond dose of >50.4 Gy
- 58. Brachytherapy in Carcinoma Esophagus • Iridium 192 is used – high dose rate brachytherapy is used most commonally • Insertion- Transnasal or transoral route • 1cm proximal and 1 cm distal margin is taken based on pretreatment tumor length.
- 59. Ca Esophagus -Brachytherapy indications Good candidates Poor candidates Contraindications Primary tumor length ≤ 10 cm length Primary tumor length> 10 cm length TE fistula Tumor confined to esophageal wall Extra –esophageal extension Cervical esophagus location Thoracic esophagus location Regional Lymphadenopathy Stenosis which cannot be bypassed No nodal / systemic metastasis Tumor involving EGJ or cardia
- 60. Definitive brachytherapy Palliative brachytherapy
- 61. Palliation • 60 -80% rate of relief from dysphagia with radiation. • MC received shedules were 20Gy/5# , 30Gy/10# , 35Gy/15# (with conc cisplatin or FU) better relief of dysphagia in CRT group. • Palliation by brachytherapy • Stent placement –inferior compared to radiation /chemotherapy
- 62. Treatment sequale • Esophagitis , dysphagia >75% , other usual symptoms nausea , vomiting , weight loss • Cytopenias with 2 drug chemoregimens are more pronounced. • Chemoradiation patients grade 3 – grade 4 toxities as high as 40%. • Late side effects :Stricture formation 14- 30% ,stenosis 60% • Rare – radiation pneumonitis (14% -grade 2) , cardiac toxicity 10% (pericardial effusion , IHD , HF) • TEF – 5-10% ( advised stenting , excision , bypass or intubation) , MS -10 weeks!
- 63. CHEMOTHERAPY
- 64. Role of Preoperative Chemotherapy • In resectable esophageal cancer (adenocarcinoma of esophagus and GE junction) • Benfits - Downstaging of disease to facilitate resection -Improvement of local control -Relief of dysphagia • The limiting thing is delay in definitive treatment with risk of further spread of disease specially in non responders • Response rate – 50% • Improve overall survival • Absolute benefit – 2 yr survival – 7% 5 yr survival - 4%
- 65. Regimens • Only for adenocarcinoma of thoracic esophagus- 2 cycles 21 days apart with • Fluorouracil – 1000 mg/m2 IV continuous infusion over 24 hr daily on D1- D4 • Cisplatin – 80 mg/m2 IV on Day 1
- 66. Postoperative Chemotherapy • Based on various trials no survival benefit was found • Disease free survival rate was improved speacially in patients who had R0 esection and was found nodepositive and can be considered for these patients • No benefit in in patients with R0 resection and N0 node Regimen • Capecitabine and Oxaliplatin Capecitabine 1000mg/m2 PO BID D1-14 Oxaliplatin 130mg/m2 IV D1 21 days cycle
- 67. Systemic Therapy for Recurrent or Metastatic disease • First line therapy -Two drug regimen is preferred (lower toxicity) • Three drug regimen reserved for medically fit patients with good performance status • Trastuzumab should be added in first line chemotherapy for HER 2 overexpressing metastatic adenocarcinoma Drug -Single agents Response rate Cisplatin 19 to 35 % Paclitaxel 25 to 35 % Docetaxel, 100 mg/m2 20-25% Irinotecan 15% Methotrexate less than 5% Etoposide less than 5% Ifosamide less than 5% Carboplatin less than 5%
- 68. Regimens used as combination chemotherapy First line therapy:- Cisplatin and fluoropyrimidines (cat1) • Cisplatin 75-100 mg /m2 IV D1 + Fluorouracil 750-1000 mg /m2 IV continuous infusion over 24 hours daily on D1-4 – 28 DAY CYCLE • Cisplatin 50 mg /m2 IV D1 + Fluorouracil 2000 mg /m2 IV continuous infusion over 24 hours daily on D1+ Leucovorin 200 mg /m2 IV on D1 (14 day cycle) • Cisplatin 80 mg /m2 IV on D1+Capecitabine 1000 mg /m2 PO BID on D1-14 (21 days cycle)
- 69. Fluoropyrimidine and Oxaliplatin:- • Oxaliplatin 85 mg /m2 IV D1 + Leucovorin 400 mg /m2 IV D1 + Fluorouracil 400 mg /m2 IV push on D1 and 1200 mg /m2 IV continuous infusion over 24 hr on D1&2 – (14 days cycle) • Oxaliplatin 85 mg /m2 IV D1 + Leucovorin 200 mg /m2 IV D1 + Fluorouracil 2600 mg /m2 IV continuous infusion over 24 hr on D1 – (14 days cycle) • Capecitabine 1000 mg /m2 PO BID D1-14 + Oxaliplatin 130 mg /m2 IV D1 (-21 days cycle) OTHER REGIMENS – DCF modifications- Docetaxel + cisplatin+ fluorouracil (OR) Docetaxel + oxaliplatin+ fluorouracil (OR) Docetaxel + carboplatin+ fluorouracil Paclitaxel with cisplatin or carboplatin Epirubicin + cisplatin + fluorouracil
- 70. TRASTUZUMAB • Used as first line therapy in recurrent or metastatic adenocarcinoma esophagus overexpressing HER2 • When compared with 1st line combination chemotherapy, survival advantage (13.8 vs 11.1 months) and response rate (47 vs 35%). • Dosage – with chemotherapy • 8 mg/kg IV loading dose on D1 of cycle 1 then 6mg/kg IV every 21 DAYS OR • 6mg/kg IV loading dose on D1 of cycle 1 then 4mg/kg every 14 DAYS
- 71. Second line therapy • Ramucirumab + Paclitaxel ( Cat 1 for EGJ adenocarcinoma ) (Ramucirumab 8mg/kg IV on D1&15 + Paclitaxel 80 mg /m2 IV on D1,8,15 – 28 days cycle) • Ramucirumab (Cat1 foe EGJ adenocarcinoma) – 8mg/kg IV on D1 – 14 days cycle • Docetaxel – 75-100 mg /m2 IV on D1 – 21 days cycle • Paclitaxel – 135-250 mg /m2 on D1 – 21 days cycle • Paclitaxel – 80 mg /m2 IV on D1,8,15 cycled every 28 days • Other regimens – irinotecan , irinotecan+fluorouracil, irinotecan +cisplatin, docetaxel + irinotecan
- 72. Follow up and Surveillance Tumor classification Therapy used Recommendations Tis Endoscopic resection(ER)/Ablat ion EGD X 6mnthly for 1-2 yr, then annually for 3 more yrs. Esophagectomy EGD based on symptoms. If incomplete resection then EGD every 6 mnths for 1-2 yr T1a with or without BE ER/Ablation EGD every 3 mnthlyx1yr then every 4-6 mnths for 2nd yr then annually for 3 more yrs T1a Esophaegctomy EGD based on symptoms. If incomplete resection then ablation f/b EGD every 3 mnthlyx1yr then every 4-6 mnths for 2nd yr then annually for 3 more yrs T1b , N0 ER/Ablation EGD every 3 mnthlyx1yr then every 4-6 mnths for 2nd yr then annually for 3 more yrs. Further surveillance will depend on relapse. PET-CT OR CT chest & abdomen yearly x 3yrs and then if needed clinically
- 73. Follow up and Surveillance T1b ANY N Esophagectomy PET-CT or CT chest and abdomen with contrast every 6 mnthly x 3yrs, then if clinically warranted. If incomplete resection then ablation f/b EGD every 3 mnthlyx1yr then every 4-6 mnths for 2nd yr then annually for 3 more yrs Chemoradiation (non surgical candidates) EGD 6-12 months x 2 yrs then annually x3 yrs PET-CT OR CECT chest and abdomen every 6-9 months x 2yrs then annually upto 5 yrs Chemoradiation (cadidate of salvage esophagectomy) EGD 6-12 months x 2 yrs then annually x3 yrs PET-CT OR CECT chest and abdomen every 6-9 months x 2yrs then annually upto 5 yrs T2-T4,N0- N+,T4b Bimodality therapy (chemoradiation) EGD every 3-4 mnthly x2 yr, every 6 monthly for 3rd yr , then as clinically warranted. PET-CT OR CECT chest and abdomen every 6-9 months x 2yrs then annually upto 5 yrs T2-T4,N0- N+,T4b Trimodality therapy Imaging (PET-CT,CECT) every 4-6 monthly X 1 yr then 6-9 monthly for next 2 yr.
- 74. SUMMARY • Often diagnosed in late stages, so poorer outcomes. • Multimodality treatment is recommended and essential for carcinoma esophagus. • Pattern of failure - ~50% local failures needs improved local treatment . • 75% succumb to distant metastasis as well , Molecular markers and newer targeted therapies under study.
- 75. THANK YOU !!
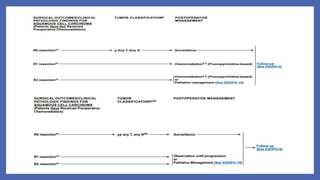
- 76. NCCN 2019 -SCC
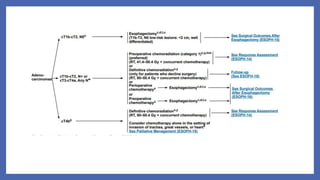
- 79. Adenocarcinoma