Chapter 14 - Gases
- 2. Simple kinetic molecular theory is used to explain gas behavior. You may have already observed some everyday examples of gas behavior. For example, you may have noticed that a sealed bag of potato chips bulges at its seams when placed in a sunny window. The air inside the bag exerts greater pressure as its temperature increases. Why does this happen? Kinetic theory can explain this and other interesting gas behaviors. Kinetic Theory
- 3. The kinetic theory of gases makes several basic assumptions. It assumes that gases consist of hard, spherical particles, usually atoms or molecules, that have the following properties. First, the gas particles are so small in relation to the distances between them that their individual volumes can be assumed to be insignificant. The large relative distances between the particles means that there is considerable empty space between the particles.
- 4. This assumption that gas particles are far apart explains the important property of gas compressibility: A gas is easily compressed because of the space between the particles . The second property of gas particles assumed by the kinetic theory is that no attractive or repulsive forces exist between the particles. As a result, gases are free to move inside their containers. In fact, a gas expands until it takes the shape and volume of its container.
- 5. The third assumption is that gas particles move rapidly in constant random motion. The particles travel in straight paths and move independently of each other. Only when a particle collides with another particle or object does theory assumes further that these collisions between gas particles are perfectly elastic, which means that during a collision the total amount of kinetic energy remains constant and that the kinetic energy is transferred without loss from one particle to another.
- 7. Gas particles in constant random motion No loss of K.E.
- 8. Four variables are generally used to describe a gas. The variables and their common units are pressure ( P ) in kilopascals, volume ( V ) in liters, temperature ( T ) in kelvins, and number of moles ( n ). The gas laws will enable you to predict gas behavior at specific conditions. Understanding the gas laws will help you understand everyday applications of gases in automobile airbags, scuba-diving equipment, and hot-air balloons, among many others. Variables That Describe a Gas
- 9. Using the kinetic theory, you can predict and explain how gases will respond to a change of conditions, specifically the pressure. When you pump up a tire, you should expect the pressure inside it to increase. Collisions of gas particles with the inside walls of the tire result in the pressure that is exerted by the enclosed gas. By adding gas, you increase the number of gas particles, thus increasing the number of collisions, which explains why the gas pressure increases. Amount of Gas
- 10. As long as gas temperature does not change, doubling the number of gas particles doubles the pressure. Tripling the number of gas particles triples the pressure, and so forth . Once the pressure exceeds the strength of the container, however, the container will rupture.
- 11. In a similar way, letting the air out of a tire decreases the pressure inside the tire. The fewer particles inside exert less pressure. When a sealed container of gas under pressure is opened, gas inside moves from the region of higher pressure to the region of lower pressure outside. This is the principle used in aerosol cans.
- 12. There are other ways to increase gas pressure. You can raise the pressure exerted by a contained gas by reducing its volume. The more the gas is compressed, the greater is the pressure it exerts inside the container. Reducing the volume of a contained gas by half doubles the pressure . Doubling the volume of the container will halve the gas pressure because the same number of gas particles occupy a volume twice the original size. Volume
- 14. Temperature
- 15. Raising the temperature of an enclosed gas provides yet another way to increase gas pressure. The faster-moving particles impact the walls of their container with more energy, exerting greater pressure. If the average kinetic energy of a gas doubles, the Kelvin temperature doubles and the pressure of the enclosed gas also doubles. By contrast, as the temperature of an enclosed gas decreases, the particles move more slowly and have less kinetic energy. They strike the container walls with less force. Halving the Kelvin temperature of a gas in a rigid container decreases the gas pressure by half.
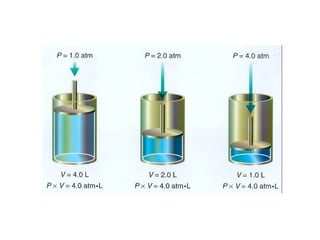
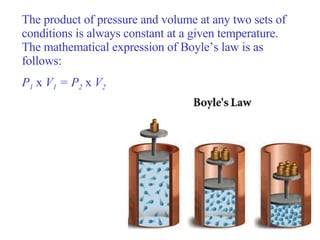
- 17. Consider the effect of pressure on the volume of a contained gas while the temperature remains constant. When the pressure goes up, the volume goes down. Similarly, when the pressure goes down, the volume goes up. The first person to do a systematic quantitative study of this pressure-volume relationship was the Anglo-Irish chemist Robert Boyle (1627-1691). In 1662, Boyle proposed a law to describe this behavior of gases. Boyle’s law states that for a given mass of gas at constant temperature, the volume of the gas varies inversely with pressure. In an inverse relationship, the product of the two variables quantities is constant.
- 18. The product of pressure and volume at any two sets of conditions is always constant at a given temperature. The mathematical expression of Boyle’s law is as follows: P 1 x V 1 = P 2 x V 2
- 19. Example: A balloon contains 30.0 L of helium gas at 103 kPa. What is its volume when its pressure is reduced to 25.0 kPa? Boyle’s Law: Solution: 1. Rearrange Boyle’s Law to find unknown, V 2 . 2. V 2 = V 1 x P 1 / P 2 3. V 2 = 30.0 L x 103 kPa / 25.0 kPa = 1.24 x 10 2 L P 1 x V 1 = P 2 x V 2 Volume Pressure
- 20. The Temperature-Volume Relationship: Charles's Law
- 21. In 1787, the French physicist and balloonist Jacques Charles (1746-1823) investigated the quantitative effect of temperature on the volume of a gas at constant pressure. In every experiment, he observed an increase in the volume of a gas with an increase in temperature, and a decrease in volume with a decrease in temperature. From his quantitative studies, Charles observed that at constant pressure the graph of gas volume versus temperature yields a straight line. In addition to the straight lines, another important feature emerged. The lines extended (extrapolated) to zero volume ( V = 0) all intersect the temperature axis at the same point, -273.15°C.
- 23. William Thompson (Lord Kelvin) realized the significance of this temperature value. He identified -273.15°C as absolute zero, the lowest possible temperature. It is the temperature at which the average kinetic energy of gas particles would theoretically be zero. This was the basis for the absolute temperature scale established by Kelvin in 1848. This scale is now called the Kelvin temperature scale. On the Kelvin temperature scale, 0 K corresponds to -273.15°C.
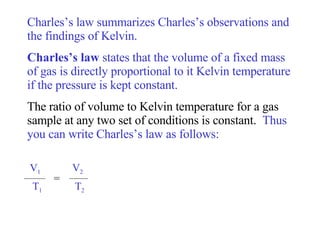
- 24. Charles’s law summarizes Charles’s observations and the findings of Kelvin. Charles’s law states that the volume of a fixed mass of gas is directly proportional to it Kelvin temperature if the pressure is kept constant. The ratio of volume to Kelvin temperature for a gas sample at any two set of conditions is constant. Thus you can write Charles’s law as follows: V 1 V 2 T 1 T 2 =
- 26. Gay-Lussac’s Law
- 27. Example: A balloon inflated in a room at 24 ºC has a volume of 4.00 L. The balloon is then heated to a temperature of 58ºC. What is the new volume? Solve: Use Charles’ Law (V 1 /T 1 = V 2 /T 2 ) to solve for unknown, V 2 . Temperature must always be express in kelvin. T 1 = 24ºC + 273 = 297 K T 2 = 58ºC + 273 = 331 K = 4.46 L V 2 = V 1 x T 2 T 1 = 4.00 L x 331 K 297 K
- 28. The Temperature-Pressure Relationship: Gay-Lussac's Law
- 29. On a hot summer day, the pressure in a car tire increases. This increase illustrates a relationship that was discovered in 1802 by Joseph Gay-Lussac (1778-1850), a French chemist. Gay-Lussac’s law states that the pressure of a gas is directly proportional to the Kelvin temperature if the volume remains constant. Therefore assuming that the volume remains constant, you can write Gay-Lussac’s law as follows: P 1 P 2 T 1 T 2 =
- 30. Example: The gas in an aerosol can is at a pressure of 103 kPa at 25 ºC. What will the pressure be when the temperature reaches 928ºC? Solve: Use Gay-Lussac’s law (P 1 /T 1 = P 2 /T 2 ) to solve for the unknown, P 2 . First convert Celsius temperatures to kelvins. T 1 = 25ºC + 273 = 298 K T 2 = 928ºC + 273 = 1201 K Rearrange to isolate P 2 . = P 2 = P 1 x T 2 T 1 103 kPa x 1201 K 298 K
- 31. THE COMBINED GAS LAW If you have been wondering how to remember the individual expression for the gas laws, there is really no need. A single expression, called the combined gas law , combines the three gas laws, as follows: P 1 x V 1 P 2 x V 2 T 1 T 2 =
- 32. The other laws can be obtained from this law by holding one quantity (pressure, volume, or temperature) constant. For example, suppose you hold temperature constant. Rearrange the combined gas law to get the two temperature terms on the same side of the equation and then cancel. P 1 x V 1 = P 2 x V 2 As you can see, you are left with Boyle’s Law. The same kind of process yields Charles’s law when pressure remains constant and Gay-Lussac’s law when volume remains constant. P 1 x V 1 = P 2 x V 2 x T 1 T 2
- 33. IDEAL GAS LAW Up to this point, you have worked with three variables regarding gas behavior: pressure, volume, and temperature. There is a fourth variable still to be considered: the amount of gas in the system, expressed in terms of the number of moles . Suppose you want to calculate the number of moles ( n ) of a gas in a fixed volume at a known temperature and pressure. The calculation of moles is possible by modifying the combined gas law. You can understand the modification by recognizing that the volume occupied by a gas at a specific temperature and pressure must depend on the number of gas particles.
- 34. The number of moles of gas is directly proportional to the number of particles . Hence, moles must be directly proportional to volume as well. Therefore, you can introduce moles in to the combined gas law by dividing each side of the equation by n . This equation shows that (P x V)/(T x n ) is a constant. This constancy holds for what are called ideal gases. A gas behaves ideally if it conforms to the gas laws. P 1 x V 1 P 2 x V 2 T 1 x n 1 T 2 x n 2 =
- 35. If you could evaluate the constant (P x V)/(T x n ), you could then calculate the number of moles of gas at any specified value of P, V, and T. This constant is symbolized as R . You can find the actual value of R , given an important fact about gases: 1 mol of every gas occupies 22.4 L at STP. Inserting the values of P, V, T, and n into the equation: The ideal gas constant ( R ) has the value 8.31 L x kPa/K x mol . Rearranging the equation for R , you obtain the usual form of the ideal gas law: R = = = 8.31 L x kPa/K x mol P x V T x n 101.3 kPa x 22.4 L 273 K x 1 mol
- 36. P x V = n x R x T ; or, PV = nRT An advantage of the ideal gas law over the combined gas law is that it permits you to solve for the number of moles of a combined gas when P, V, and T are known . P is the pressure V is the volume n is the amount of gas (moles) R is the Real Gas constant, with units appropriate for the units of pressure, volume, temperature, and amount of gas. T is the temperature (in Kelvin because an absolute scale is necessary.)
- 37. Example: A deep underground cavern contains 2.24 x 10 6 L of methane gas (CH 4 ) at a pressure of 1.50 x 10 3 kPa and a temperature of 315 K. How many moles of CH 4 does this cavern contain? Solve: Calculate the number of moles ( n ) using the ideal gas law. Rearrange the equation for the ideal gas law to isolate n . = = 1.28 x 10 6 mol CH 4 n = P x V R x T 1.50 x 10 3 kPa x 2.24 x 10 6 L 8.31 x 315 K L x kPa K x mol
- 38. Real vs. Ideal Gases An ideal gas is one that follows all of the assumptions of the kinetic theory. Its particles could have no volume, and there could be no attraction between particles in the gas. Unfortunately, there is no gas where this holds true. At many conditions of temperature and pressure, however, real gases do behave like ideal gases. In a real gas the particles do have volume, and there are attractions between the particles. Because of these attractions, gases can condense, or even solidify, when it is compressed and cooled.
- 39. AVOGADRO’S HYPOTHESIS p. 300 The particles that make up different gases are not the same size. For example, chlorine molecules have a large number of electrons, protons and neutrons. They are bigger and occupy more volume than hydrogen molecules, which have only two protons and two electrons. Early scientists assumed that collections of larger molecules must have larger volumes than collections of an equal number of small molecules .
- 40. Thus many scientists disbelieved when they heard of Avogadro’s hypothesis in 1811: Equal volumes of gases at the same temperature and pressure contain equal numbers of particles. It was as if Avogadro was suggesting that two rooms of the same size could be filled by the same number of marbles or basketballs. Avogadro realized that there would be large expanses of space between the particles since they were so small and far apart. At STP (0 º C, 101.3kPa), 1 mol (6.02 x 10 23 ) of particles of any gas, regardless of the size of the particles, occupies 22.4 L. Thus whenever you have equal volumes of gases at the same temperature and pressure, the volumes should contain equal numbers of particles.
- 41. DALTON’S LAW The particles in a gas mixture at the same temperature have the same average kinetic energy. Gas pressure depends only on the number of gas particles in a given volume and on their average kinetic energy - the kind of particle is unimportant. Each particles makes the same contribution to the pressure. Thus if you know the pressure exerted by each gas in a mixture, you can add the individual pressures to get the total gas pressure.
- 42. The contribution each gas in a mixture makes to the total pressure is called the partial pressure exerted by that gas. In a mixture of gases, the total pressure is the sum of the partial pressures of the gases. P total = P 1 + P 2 + P 3 + … This equation is one mathematical form of Dalton’s law of partial pressure: At constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the component gases. The fractional contribution to pressure exerted by each gas in a mixture does not change as the temperature, pressure, or volume changes.
- 43. This fact has important implications for aviators and mountain climbers. For example, on top of Mount Everest, the total atmospheric pressure is reduced to 33.73 kPa (about one-third of its value at sea level). The partial pressure of oxygen is reduced by the same factor, to only 7.06 kPa (one-third its value at sea level).
- 44. GRAHAM’S LAW When you open a perfume bottle inside a room, the perfume molecules eventually spread throughout the room, and you smell them everywhere. Diffusion is the tendency of molecules to move toward areas of lower concentration until the concentration is uniform throughout. Thomas Graham, a Scottish chemist, did much of the early work on the study of effusion and diffusion in the 1840’s. Effusion is the process in which a gas escapes through a tiny hole in its container
- 45. Graham noticed that gases of lower molar mass effuse faster than gases of higher molar mass. From his observations, he proposed Graham’s law of effusion: The rate of effusion of a gas is inversely proportional to the square root of the gas’s molecular mass. Subsequently, this relationship was also shown to be true for the diffusion of gases. Thus the rate of diffusion of a gas is also inversely proportional to the square root of its molar mass. To understand this, remember that if two bodies of different masses have the same kinetic energy, the lighter body must move faster (KE = 1/2mv 2 ).
- 46. The gas of lower molar mass should therefore diffuse and effuse faster. It is easy to show this with a balloon filled with air and one filled with helium. There are pores in a balloon that are large enough to allow both helium atoms and molecules of air to pass through freely. Balloons filled with air stay inflated longer, because the air molecules are more massive than helium atoms. The air particles move more slowly; therefore they diffuse and effuse more slowly than helium atoms. The fast-moving helium atoms rapidly effuse through the pores in the balloon. The rate of effusion or diffusion is related only to the particles speed.
- 47. In a mathematical form, Graham’s law can be written as follows for two gases, A and B. In other words, the rates of effusion of two gases are inversely proportional to the square roots of their molar masses. Heavier gas Heavier gas