Gas Chromatography by Dr. A. Amsavel
- 1. Gas Chromatography Gas Chromatography Dr. A. Amsavel, M.Sc., B.Ed., Ph.D. Principle, Instrumentation and Application
- 2. What is Chromatography? • Chromatography is a separation technique used for Qualitative and Quantitative Analysis. • Essential for testing of chemicals in An Industries • Technique developed in 1941 by Martin & Synge and were awarded Nobel Prize in 1952 (partition chromatography) awarded Nobel Prize in 1952 (partition chromatography) • In 1951 first GC experiment was performed by Martin & James • In 1955, the first commercial apparatus for gas-liquid chromatography appeared on the market • Around mixture of 200 related compounds can be tested in preset GCs
- 3. Chromatographic Separation Chromatography is based on a Physical equilibrium that results when a solute is transferred between the mobile and a stationary phase. K = Distribution coefficient or Partition ratio K = CS/CM • CS -Molar conc. of the solute in the stationary phase • CM -Molar conc. of the solute in the mobile phase.
- 4. Gas Chromatography Gas Chromatography is a chromatographic technique. Where Gas is used as Mobile phase. Stationary phases are two types; Solid or Liquid • Gas-Solid Chromatography (GSC ): • Gas-Solid Chromatography (GSC ): Separation is based on physical adsorption , but not widely used. • Gas-Liquid Chromatography (GLC ): GLC - Separation is based partition between mobile phase (MP) and Stationary Phase (SP). Used extensively.
- 5. Separation in GC • In a mixture, each component has a different distribution coefficient, and thus spends a different amount of time absorbed on solid or partition on liquid stationary phase and moving carrier gas • The sample then has the opportunity to interact with the stationary phase as it moves past it. stationary phase as it moves past it. Samples that interact greatly, which move more slowly. Samples that interact weakly, which move more quickly. Because of this difference in rates, the samples can then be Separated into their components.
- 6. GC : Separation Principle • Compounds that have a greater affinity for the stationary phase spend more time in the column and thus elute later and have a longer retention time (Rt). • Affinity for the stationary phase is based on intermolecular interactions and the polarity of the stationary phase.
- 7. Advantage of GC GC is used for separating and analyzing the volatile compounds. o Advantage o Efficiency and analysis speed: o Easy and fast o Accuracy and reproducibility o Low sample quantity (µg/ng) / low detection level o Can test wide range of organic compounds o High resolution and High Sensitivity (ppm) – Used to analyze azeotropic mixtures and samples with close boiling points, some isotopes, cis-trans isomers, adjacent or inter trans isomers, optical isomers, etc.
- 8. Definition • Carrier Gas : Mobile phase or carrier gas. Carrier gas in GC is to move the solutes along the column. • Retention time. is defined as the time elapsed between the injection of the sample and the appearance of the maximum peak response of the eluted sample zone. • Relative retention (RRT): Relative Retention time of a component is • Relative retention (RRT): Relative Retention time of a component is relative to that of another used as a reference, obtained under identical conditions. • Resolution (RS): The resolution is the separation of two components in a mixture • Make-up gas. Career gas flow at detector (20-40 mL/m) is provided for maximum performance of detector. This additional flow (make-up gas) is adjusted at detector. Carrier gas and make up gas is mixed at the outlet of the column and enters to the detector.
- 9. Definition • HETP- It is the distance on the column in which equilibrium is attained between the solute in the gas phase and the solute in liquid phase. Larger the number of theoretical plates/ smaller the HETP, the more efficient the column for separation. HETP = Length of column/n ; Where n = number of theretical plates= 16 * x2/y2 • Retention Volume: (1) VR = tR ×F (retained) (2) VM = tM ×F (non- retained) • WCOT: Wall-coated columns are capillary tubes coated with a thin layer of the liquid stationary phase • SCOT: Support-Coated Open Tubular columns have finely divided layer of solid supported material deposited (~30 mm) on the inner wall, on to surface of the capillary on which the liquid stationary phase is coated
- 10. Definition Number of theoretical plates (N): A measure of column efficiency. For Gaussian peaks, it is calculated by: N = 16(tR/W) 2 where tR is the retention time of the substance, and W is the peak width at its base Resolution (RS): The resolution is the separation of two components in a mixture, calculated between peaks (1&2); tR - Retention time & W – peak width RS = 2 × (tR2 - tR1)/(W1 + W2) Symmetry factor (AS): Also known as the “tailing factor”, of a peak is calculated by: A = W /2f (Fig-1) calculated by: AS = W0.05/2f (Fig-1) Where W-0.05 is the width of the peak at 5% height from base and f is the distance from the peak maximum to the leading edge of the peak. The signal-to-noise (S/N) ratio: is a useful system suitability parameter. The S/N ratio is calculated : S/N ratio = 2H/h ) Fig-2 Fig-1 Fig-2
- 11. GC Instrumentation Gas Chromatograph consists: 1. Carrier Gas source 2. Injection Port / Injector 3. Column Oven and Column 3. Column Oven and Column 4. Detector & 5. Data processing/Software.
- 13. GC Instrumentation GC Parts and It’s Function: • The injection port, column, and detector are temperature controlled and can be modified while analysis . Temperature can be optimized as desired for better separation, resolution and peak symmetry • Carrier gas : Helium, Nitrogen, or hydrogen, depending on the • Carrier gas : Helium, Nitrogen, or hydrogen, depending on the column and detector in use. • Column is brain or heart of the GC. It determines the separation of compounds • The type of detector used depends on the nature of the compounds analyzed • Detector output is recorded as a function of time, and the instrument response, measured as peak area or peak height are proportional to the amount present
- 14. GC: Injector • Injector is an Inlet of the sample to the Gas chromatograph. Carrier gas continuously flows into the injector. • Injector is maintained at elevated temperature . Higher than boiling point of samples (normally 100 – 300 °C). End of syringe • It operates in Split or Splitless mode. • Injector vaporize the injected sample and mix with the carrier gas and flows to the column. • In case of split mode (capillary columns), injector allows proportion (as per slit ratio) of carrier gas and sample vapour to the column. Remaining portion exit from the split outlet, which is regulates by needle valve Connected to column
- 15. Sample Injection • Fill sample Liquid or vapor in micro syringe. • Overfill syringe then adjust to desired amount (0.1-1µL and inject. • Do not let the sample remain in the syringe long before injecting to avoid vapor loss/ low boiling will escape. injecting to avoid vapor loss/ low boiling will escape. • Hold the head of the syringe during injection. While piercing the septa , plunger may be pushed up due to pressure. • Inject as close as possible to the column head. • Push the plunger fairly rapidly during injection.
- 16. GC column Oven • GC oven capacity designed to hold one or two columns and which can heat up to more than 400°C. • Oven can be heated or cooled a rapidly. Also designed the control temperature and set gradient with 1-100°C/min. • The temperature can be controlled ± 0.1 C to get reproducible separations. • Also can operate at low temperature with cryogenic (use of liq. N2 or CO2) • Set Isothermal or programmed temperature modes as required. • Isothermal: Same temperature throughout the analysis. • Programmed: To get desired separation, alter or program the oven temperature (column). Set initial temperature (hold if required), increase rate of temperature change (ramp), final temperature, and hold time at the final temperature. Eg.100°C hold 5 min ramp 10°C/min to 250°C hold for 10 min
- 17. Oven Temperature & Separation • Oven temperature should be above the boiling point and below the degradation temperature of solute. • Separations of compounds are temperature dependent, hence oven temperature (Column) is important. Isothermal oven: The oven temperature is constant throughout the analysis. o The oven is always ready for a sample analysis. There is no recovery time between analyses. o There is no recovery time between analyses. Programmed oven: The oven temperature changes, usually upward, during the analysis. o Analysis time is reduced by proper set temperature o Achieve good Peak separation/ resolution & peak shapes and faster analysis of complex molecules. o Disadvantage is components are subjected to higher temperatures, may cause degradation of sensitive components. Excess time for cooling of oven
- 18. Capillary Column Fused silica used in capillary columns. It is inert and robust. A capillary column is an open tube coated with the stationary phase on its inside surface. Fused silica tube wall Columns Inside Diameter (ID) range from 0.1mm to 0.53 mm Length is 30 meters to 100 meters Theoretical plates up to 300000 . Work with Split less (entire sample) or Split (part of sample divided before it enters the column). Liquid phase Column ID (2r) Film thicknes Fused silica tube wall
- 19. Packed Column Stationary phase is coated on a finely-divided inert material packed into a metal, glass, or plastic tube 1/8- or 1/4-inch outside diameter. Length 1-5 meters Stainless steel—durable, but a reactive surface , may cause component Stainless steel—durable, but a reactive surface , may cause component loss or peak tailing. Glass (deactivated surface) better peak shape, but fragile Teflon tubes are also used 4000 theoretical plates Low resolution and limited separation & use
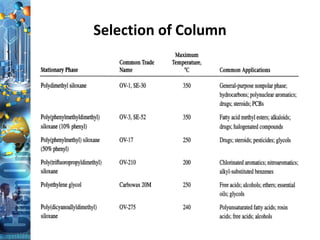
- 20. Stationary Phase Chemical nature and functional groups: • Polydimethyl siloxane —Non-polar to moderately polar • Phenyl methyl silicones (5 to 50% phenyl)—Moderately polar to polar • Polyglycol and Cyanopropyl phenyl-Very polar
- 21. Carrier Gas flow GCs provide electronic pneumatic control (EPC).
- 23. Polarity of Stationary Phase 100% dimethyl polysiloxane 5% phenyl - arylene - 95% dimethyl polysiloxane 5% diphenyl/95% dimethyl polysiloxane 20% diphenyl/80% dimethyl polysiloxane 6% cyanopropylphenyl/94% dimethyl polysiloxane 35% diphenyl/65% dimethyl polysiloxane Polarity based on ionic liquid. Number is calculated using McRaynold constant Trifluoropropylmethyl polysiloxane 50% diphenyl/50% dimethyl polysiloxane 14% cyanopropylphenyl/86% dimethyl polysiloxane 50% cyanopropylphenyl/50%Phenylmethyl polysiloxane Polyethylene Glycol
- 25. Column Selection Criteria Column Selectivity is based on Chemical nature of stationary phase. Low selectivity: Elute together. High selectivity: Peaks separate well. GC Column Selection Parameters o Dimensions : Column length , ID, film thickness o Dimensions : Column length , ID, film thickness o Conditions : temperature , flow rate o Composition –Stationary phase composition, carrier gas o Phase selection : Principle that "like dissolves/separates like. o A non-polar column for analyses of non-polar compounds. o A polar columns for the separation of polar compounds. Note: Bonded phases are preferred. Immobilized and/or chemically bonded (cross linked) within the column.
- 26. Retention Mechanism of Column • Non-polar columns are primarily dispersive. Based on Van-der Waals forces. Intermolecular attraction between Stationary phase and compound. Larger size/ compounds with higher boiling points have longer retention. Phases with phenyl functional groups can also undergo a moderate amount of π - π interac ons. Elu on order is based on boiling points. • Intermediate polar and polar columns are strongly dispersive. Moderate amounts of hydrogen bonding and basic interactions. Phases with phenyl functional groups can also undergo π - π dipole- dipole, and dipole-induced dipole interactions. Phases with Cyanopropyl groups undergo strong dipole-dipole and moderate basic interactions. • Highly polar columns are strongly dispersive, very strongly dipole- dipole, and very strongly dipole-induced dipole. Moderately basic interactions are also possible.
- 27. Column Efficiency Internal Diameter (mm) Efficiency: Plates/Meter (N/m) Efficiency: Total Plates (N) Capacity Each Analyte (ng) 0.53 1,300 39,000 1000-2000 0.32 2,300 69,000 400-500 0.32 2,300 69,000 400-500 0.25 2,925 87,750 50-100 0.20 3,650 109,500 <50 0.18 4,050 121,500 <50 0.10 7,300 219,000 <10 Note: Theoretical values for 30 meter columns, calculated @ a k = 6.00 and 85% coating efficiency
- 28. Influence of Column ID • The efficiency ie plates (N) or plates per meter (N/m) increases as the ID of the column decreases. • Efficiency (number of theoretical plates) and sample capacity (amount enters into column) sample capacity (amount enters into column) determines the separation. – Less sample -Sharp peak, but low detection level – Higher sample may lead to overload/broad peak • Optimize either efficiency or sample capacity to get narrow and well-resolved peaks.
- 29. Effects of Column ID. Column Length (m) Inlet Pressure (psi) Peak 1 Retention (min) Peak 1/2 Resolution (R) Efficiency: Total Plates (N) 15 5.9 8.33 0.8 43,875 30 12.0 16.68 1.2 87,750 60 24.9 33.37 1.7 175,500 Note: Theoretical values for 0.25 mm ID columns with 85% coating efficiency, 145 °C isothermal analyses, helium at 21 cm/sec, k (peak 1) = 6.00
- 30. Attributes of Column ID ID-0.10mm - 0.20mm ID -0.25mm -0.32mm ID 0.53mm Characteristics • Highest efficiency • Shorter analysis time • Lower sample loading capacity Characteristics • High efficiency • Good performance for analysis time and sample loading capacity Characteristics • Good efficiency • Longer analysis time • Higher sample loading capacity • May require higher flow rates capacity loading capacity • May require higher flow rates than MS detectors can tolerate Application • Highly complex samples • Fast GC • GC-MS • Split injection Application • Complex samples • Wide concentration range • Split, splitless, direct, headspace Application • Packed column replacement • Purity analysis • Split, splitless, direct, headspace As inner diameter increases, efficiency decreases, sample loading capacity increases, optimal flow rate increases, and analysis me increases.
- 31. Influence of Column Length Column length • Longer columns provide greater resolution, but increase back pressure • To increasing resolution is to reduce column ID • Column resolution is proportional to the square root of • Column resolution is proportional to the square root of column length. Factor to consider : • Shorter columns are recommended when great resolution is not required, such as for screening purposes • Longer Columns Increase Cost and Analysis Time
- 32. Impact of Film Thickness Optimal film thickness should be depending on the application. Most 0.25 mm ID columns have a 0.25 or 0.50 µm film thickness. Decreasing Film Thickness (0.1 µm to 0.5µm) Peaks are sharper in lower film thickness. High resolution and increase signal-to-noise. Reduced column bleed, can use of max. operating temperature. Elute with shorter retention times and at lower temperatures. Useful: Medium and high molecular weight compounds Increasing Film Thickness (1 µm to 10µm) Reduce the analyte-tubing interaction Increased sample capacity and Increased analyte retention. (good- purity testing) The drawback is increased peak widths (less resolution) Increased column bleed, and a reduced maximum operating. Useful: Volatile, low molecular weight compounds
- 33. Attributes of Film Thickness 0.1 µm to 0.5µm 1 µm to 10µm Characteristics • Shorter retention times • Lower bleed • Higher maximum temperatures • Lower sample loading capacity Characteristics • Longer retention times • Higher bleed • Lower maximum temperatures • Higher sample loading capacity • Lower sample loading capacity • High resolution for high molecular weight compounds • Higher sample loading capacity • High resolution for volatiles and low molecular weight compounds Applications Medium and high molecular weight compounds Applications • Volatile, low molecular weight compounds • High concentration samples (e.g., purity testing)
- 34. Detectors • Detectors sense the separated components and provide a signal. Selection of detectors based on the compounds • Detectors are destructive or non-destructive of the analytes. • All the detectors are either concentration-dependent or mass dependant. Can connect multiple detectors for good response • The detector set with correct temperature to prevent decomposition. decomposition.
- 35. Flame Ionization Detector (FID) • The carrier gas from the column mixes with hydrogen and is burned (flame) in air • Carbon-containing material enters from column to the flame generated the ions / charged particles • FID consists two electrodes, one is the jet where the flame burns, another electrode is wire / grid kept tip of the flame as collector. the flame burns, another electrode is wire / grid kept tip of the flame as collector. • When charged particles enters between the electrode (pd of 100 to 300 V), collector collects the ions and current raises as per concentration. • After amplification of the week current, it creates the chromatogram. • FID can be used only for the compound, which can creates ions in a flame.
- 36. Thermal Conductivity Detector (TCD) TCD is non-destructive detector, working based on the thermal conductivity of gas mixtures as a function of their composition The detector consists two identical thermistors (filaments) and maintained at a constant temperature ( for ref and sample from column) Both thermistors are located within the path of the carrier gas. One is flushed by the carrier gas evolving the column, while the other is from by a part of the carrier gas entering the injector ( Figure in next page) part of the carrier gas entering the injector ( Figure in next page) In the steady state a temperature equilibrium is established between thermal conductivity of the carrier gas & electrical current in the filament. When a solute elutes from the column, there is a change in the mobile phase composition, which changes its thermal conductivity. The thermal equilibrium being disrupted and change in the resistance, which is proportional to the concentration of the compound. Hydrogen & helium are suitable carrier gases for TCD since high thermal conductivity. Then all other gases ( compound)
- 37. Thermal Conductivity Detector (TCD)
- 38. Electron Capture Detector (ECD) • The electron capture detector has found wide use in environmental samples due to its very high sensitivity to halogen-containing components (eg. Herbicides & pesticides). • A radioactive isotope, usually 63Ni, in the detector cell emits beta particles. These collide with carrier cell emits beta particles. These collide with carrier gas to create showers of low-energy free electrons. Two electrodes and a polarizing voltage collect the electrons as a current. • Some molecules can capture low-energy electrons to form negative ions. • When such a molecule enters the cell, some of the electrons are captured and the collected current decreases. This change converts into signal/ peak
- 39. GC Application • Qualitative Analysis – by comparing the retention time or volume of the sample to the standard against the sample. • Retention time or relative retention time can be used for identification for eluted compound. Retention times are characteristic of the compounds they represent (but are not unique). Mass Detector gives the mass value to identify precisely. • Other detector – FTIR, NMR, Mass detector can be combined • Quantitative Analysis- Pear area / Peak height of elution peak is proportional to the quantity / concentration. Peak response is based of the detector used. • Method used for Quantitative Analysis shall be validated • Type of estimation- Area normalization, Internal standard, Calibration and standard, Standard addition and etc
- 40. Type of Samples for GC Analysis • Elemental analysis : % composition of elements C, H, N, sulfur in organic compounds… • All volatile organic compounds like Solvents , liquid reagents… • Silyl derivatives: Some of the organic compounds can be derivatized using Trimethylchlorosilane to re reduce the derivatized using Trimethylchlorosilane to re reduce the boiling point and test. Eg. Glucose, fructose, sorbitol etc. • Residual solvent from the solid material using Headspace samples with GC • Compound can be converted to the volatile coordination complex and tested by GC
- 42. The following Section is Applicable for Pharmaceutical Industries. Pharmaceutical Industries.
- 43. Analytical Method • Method should be demonstrate specificity, linearity, accuracy, robust, rugged an sensitive, . • Ensure that Analytical method used is validated fo meet the above characteristics. meet the above characteristics. • If test method is as per monograph, ensure that analytical method is verified for its suitability Eg USP General Chapter <1226> • Any change in the test condition shall be within the allowable limit of Pharmacopeia (next page)
- 44. Adjustment Allowed for GC condition Property USP General Chapter 621 Ph.Eur. Gen. Chapter 2.2.46 Column length ±70% ±70% Film thickness -50% to 100%. − 50 % to + 100% (cap. columns). Column ID ±50% ±50% Flow rate ±50% or more, provided the linear ±50% Flow rate ±50% or more, provided the linear flow velocity remains the same. Velocity adjustment is between +50% and -25% ±50% Oven temperature ±10 °C or Program- up to ±20% is permitted. (when the temperature must be changed from one value to another) ±10 °C Injection Volume The injection volume and split volume may be adjusted if detection and repeatability are satisfactory. may be adjusted, provided detection and repeatability are satisfactory
- 45. Starting GC Analysis • Ensure required Gas and pressure before starting GC • Test method is downloaded & verified • Create Sequence as per test method Eg Blank, system suitability (SS), reference solution , test, and bracketing standard . • Data file shall be continuous (do not repeat the file no/name) • Data file shall be continuous (do not repeat the file no/name) • Load the sample and run the system suitability and once SS passes run the test • After completion of the sequence, process the chromatograms • Print the method, sequence and data • Perform audit trail before batch release • Store the data and ensure the back up of data
- 46. Integration of Peaks • Do not integrate any peak by manually. • Integrate all the peaks or else as per procedure • Always use same processing method for processing of blank, standard & sample chromatograms in case of Assay & related substances, etc. • Verify the processing parameters like • Verify the processing parameters like – Threshold, – Width, – System suitability, – Peak names etc. • Save the processing method • Re-integration: – Do not re-integrate the chromatograms without documenting. – Document reason for reintegration.
- 47. Common problem in GC analysis • System failures may occur during analysis due to – System over pressure – Leak – Leak – Communication error – Failure of system suitability – Peak splitting/ negative peak – Spurious peak – Bracketing standard failure
- 48. Handling of Deviation/Failure How to handle the failure ? SOP shall be available and shall address the handling of Lab deviation/ incidents. SOP shall define clearly the deviation/ incident , reporting investigation, CAPA and documentation Record all the deviation/ incident happened in chromatographic analysis Record all the deviation/ incident happened in chromatographic analysis Process all the injections including the invalid injection and report and store the data along with Raw data. Do not omit any injection Investigate the deviation/ incident and find the root cause for the failure. Rectify the problem, take appropriate CAPA and document Repeat complete sample set of injections in case of sample injection failure
- 49. How to handle the problem if any • In case of interruption, due to power failure, computer interruption, time gap due to sample preparation or due to injection for ~4 hours and if system is in continuous state of equilibrium ; • Inject bracketing standard and proceed otherwise restart • Deviation in RT for sample or std, is > 15% of specified RT • Inhibit the peak upto void volume • Use the same integration parameter for entire set • Reprocess shall be at the same time for entire sequence
- 50. Documentation Ensure are followed contemporaneously • Instrument Use Log • Routine Maintenance Log • Problem Log • Column History Log • Preventive manitenance
- 51. Reference 1. Chemical Analysis: Modern Instrumentation Methods and Techniques Francis and Annick Rouessac and Steve Brooks 2007- John Wiley & Sons Ltd,. 2. Fundamentals of Gas Chromatography – Agilent manual 3. Guide to GC Column Selection and Optimizing Separations – Restek Separations – Restek 4. Analytical Separation Science, First Edition. Basic Overview on Gas Chromatography Columns Md. Musfiqur Rahman, A.M. Abd El-Aty, Jeong-Heui Choi, Ho-Chul Shin, Sung Chul Shin, and Jae-Han Shim 5. Vogel’s – Quantitative Chemical Analysis- 6th edition 6. Principles of Instrumental Analysis- 6th Edition- D.A. Skoog et al