ICH guidelines
- 3. Syllabus Contents ICH Guidelines: 1. Purpose, 2. Participants, 3. Process of harmonization, 4. Brief overview of QSEM, with special emphasis on Q-series guidelines, ICH stability testing guidelines 3 Dr.K.B.Gabhane
- 4. Objectives: • Upon completion of this section, student should be able to : 1. Define the term ICH and its purpose. 2. Explain how the concept of harmonization was adopted in practice 3. Describe the organizational setup of ICH 4. List and explain the steps in the harmonization process 5. Explain the important ICH guidelines 6. List the ICH Quality guideline components 7. Outline stability testing guidelines as per ICH 4 Dr.K.B.Gabhane
- 6. ICH and its purpose : ICH : International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human use. Rational : The differences in technical requirements across countries meant that drug makers had to spend lot of time and money to duplicate test procedures if they wanted to market their products at an international level. Hence A need was felt to rationalize and harmonize Purpose The body was set upto bring together representatives of pharmaceutical industry and regulatory bodies to discuss technical and scientific aspects of registration of drugs in order to have : Harmonization of technical requirements : Ensure safety, efficacy, and quality of medicines : Prevent duplication of clinical trials in humans : Minimize the use of animal testing without compromising safety and efficacy 6 Dr.K.B.Gabhane
- 11. FOUNDING MEMBERS OF ICH • United States of America • Japan • European region MEMBER COUNTRIES • EMA : European Commission and European Medicine Agency • USFDA : United States Food and Drug Administration • MHLW: Ministry of health, Labour and welfare, Japan REGULATORY REPRESENTATIVES • EFPIA: European federation of pharmaceutical industries association • PhRMA: USA’s Pharmaceutical Research and Manufacturers of America • JPMA : Japan’s Pharmaceutical Manufacturers Associations INDUSTRIAL REPRESENTATIVES 11 Dr.K.B.Gabhane
- 13. Process of Harmonization HARMONIZATION ACTIVITIES Formal ICH Procedure New topic for harmonization Q and A Procedure Clarification foe an existing ICH guideline Revision Procedure Adding new information to an existing ICH guideline Maintenance Procedure Changes to be made to maintain a guideline Formal ICH procedure than begins in following steps 13 Dr.K.B.Gabhane
- 14. Harmonization Process Step 1: Building Scientific Consensus Based on the objective specified, a worker group prepares a consensus (general agreement) draft called the technical document. The document further is submitted to the ICH assembly with a request for adoption Step 2 : Agreeing on the draft The draft is examined and endorsed by regulatory members of Assembly. Assembly confirms that the scientific consensus (general agreement) exist for technical issues and may proceed further for adoption. Step 3 : Consulting regional regulatory agencies Consultation, discussion and finalization of the expert draft guidelines by regulatory members at different levels: Stage 1: Draft goes to different ICH regions for discussion in their respective regulatory regions. Stage 2: Comments obtained during stage 1 are addressed by the expert working group and after discussion, consensus (general agreement) is reached to prepare the expert draft guidelines Continue………………………… 14 Dr.K.B.Gabhane
- 15. Step 3 : Consulting regional regulatory agencies Consultation, discussion and finalization of the expert draft guidelines by regulatory members at different levels: Stage 3 : This draft guidelines is finalized and signed by the ICH regulatory member experts. Step 4 : Adopting Harmonized Guidelines ICH assembly if agree that sufficient scientific consensus exists on the draft guideline, and it gets adopted as the ICH Harmonized Guideline. Step 5 : Implementing Guidelines in ICH regions ICH harmonized guideline is implemented an all the ICH regions through the respective regulatory procedures. Effective tenure is sent to ICH assembly and published on ICH website. 15 Dr.K.B.Gabhane
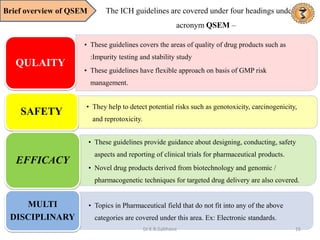
- 16. Brief overview of QSEM The ICH guidelines are covered under four headings under the acronym QSEM – • These guidelines covers the areas of quality of drug products such as :Impurity testing and stability study • These guidelines have flexible approach on basis of GMP risk management. QULAITY • They help to detect potential risks such as genotoxicity, carcinogenicity, and reprotoxicity. SAFETY • These guidelines provide guidance about designing, conducting, safety aspects and reporting of clinical trials for pharmaceutical products. • Novel drug products derived from biotechnology and genomic / pharmacogenetic techniques for targeted drug delivery are also covered. EFFICACY • Topics in Pharmaceutical field that do not fit into any of the above categories are covered under this area. Ex: Electronic standards. MULTI DISCIPLINARY 16 Dr.K.B.Gabhane
- 17. GUIDELINE SUBPART AREA COVERED Q1 Q1 A Stability testing of new drug substances and products Q1 B Photostability testing of new drug substances and products Q1 C Stability testing of new dosage forms Q1 D Bracketing and matrixing designs for stability testing of new drug substances and products. Q1 E Evaluation of stability data Q1 F Stability data package for registration applicationsin climatic zone III and zone IV. Q2 Validation of analytical procedures. QUALITY GUIDELINES The areas covered are labeled from Q1 to Q11 and deals with important aspects like Stability testing, analytical validation, impurities, quality systems, risk management, and GMP 17 Dr.K.B.Gabhane
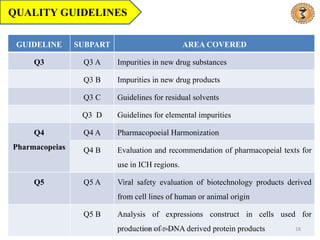
- 18. QUALITY GUIDELINES GUIDELINE SUBPART AREA COVERED Q3 Q3 A Impurities in new drug substances Q3 B Impurities in new drug products Q3 C Guidelines for residual solvents Q3 D Guidelines for elemental impurities Q4 Pharmacopeias Q4 A Pharmacopoeial Harmonization Q4 B Evaluation and recommendation of pharmacopeial texts for use in ICH regions. Q5 Q5 A Viral safety evaluation of biotechnology products derived from cell lines of human or animal origin Q5 B Analysis of expressions construct in cells used for production of r-DNA derived protein products 18 Dr.K.B.Gabhane
- 19. QUALITY GUIDELINES GUIDELINE SUBPART AREA COVERED Q5 Q5 C Stability testing of biotechnology/ biological products Q5 D Derivation and characterization of cell substrates used for production of biotechnology / biological products. Q5 E Comparability of biotechnological/biological products subject to changes in their manufacturing process Q6 Q6 A Test procedure and acceptance criteria for biotechnological /biological products. Q6 B Test procedure and acceptance criteria for biotechnological/ biological products. Q7 Good manufacturing practices for API Q8 Pharmaceutical Development 19 Dr.K.B.Gabhane
- 20. QUALITY GUIDELINES GUIDELINE SUBPART AREA COVERED Q 9 Quality risk management Q 10 Pharmaceutical Quality system Q 11 Development and manufacture of drug substances (Chemical and Biological entities) 20 Dr.K.B.Gabhane
- 21. ICH guidelines for Stability 1. These guidelines define what information must be provided at the time of applying to register a new drug molecule. 2. These guidelines were first adopted in1993 3. After revision and updation, the current version Q1A(R2) is adopted since 2003. 4. This guideline harmonizes the registration process for all drugs in the USA, Japan and EU. 5. This means a drug registered in one of these regions will not require repeated stability testing when to be sold in any of these regions. 6. The stability testing data submitted under this guideline must provide information about how the drug molecule changes over time under different storage conditions. 7. The data helps to define the ideal storage condition for the said product and the shelf life of the product. 21 Dr.K.B.Gabhane
- 22. ICH guidelines for Stability TYPES OF STABILITY TESTING : 1. Real – time testing : Testing drug product for longer duration to find out what is the maximum time for product degradation when stored under recommended storage conditions 2. Accelerated stability testing : Here product is subjected to stress in the form of higher temperatures, moisture, agitation, light, pH and packaging conditions to study its degradation profile 3. Retained sample stability testing : This involves samples that are retained from each batch that has been sent to the market 4. Cyclic temperature stress testing : Not routinely used, this involves subjecting the products to temperature stresses in a way to mimic likely market storage conditions. 22 Dr.K.B.Gabhane
- 23. Overview of ICH Stability guidelines contents : Some of the areas covered by ICH on stability testing include 1. Stress testing 2. Photostability testing 3. Batch selection for stability testing 4. Testing of container closure system ICH guidelines for Stability 23 Dr.K.B.Gabhane