Introduction Of Inactivated Poliovirus Vaccine
- 2. Epidemiology of Polio • Polio myelitis is an acute viral infection caused by poliomyelitis virus. • Three types Type 1 – most common, causes most epidemics Type 2 – most effective antigen(no serotype detected in world since 1999) Type 3 • Man is the only reservoir of infection. For every clinical case 1000 subclinical cases in children and 75 in adults.
- 3. • Infectious material – Feces and Oropharyngeal secretions. • Mode of transmission – Feco-oral(major) and Droplet(in developed countries) • IP: 7-14 days • POLIO: inapperent infection(72%) mild disease (24%) aseptic meningitis(4%) Paralytic polio(rare<1%)
- 4. POLIO IS A CRIPPLING & POTENTIALLY FATAL DISEASE HAS NO CURE
- 5. Global Scenario • GPEI(Global Polio Eradication Initiative) launched in 1988 –incidence of polio decreased by 99% from 350000 cases to 26 in 2015. • In 1988 polio endemic countries were 125, now in 2015 only 3 –Afghanistan, Nigeria, Pakistan are endemic.
- 6. Indian Scenario • Incidence in 1970 – 4,00,000 cases. • Vaccination against polio started in 1978 with EPI. • In 1985 UIP was launched. • Pulse polio Immunization programme launched in 1995. • Incidence came down to 42 cases in 2010. • Last case of polio due to Wild Polio Virus was in January 2011. • SEAR certified as polio free in March 2014.
- 7. OPV • Developed by Albert Sabin in 1961 • Live attenuated vaccine • As oral drops • Easily administered • Provides both humoral and mucosal intestinal immunity “SABIN VACCINE’’
- 8. • This intestinal immune response to opv is the main reason why mass campaigns with OPV can rapidly stop person to person transmission of wild polio virus • Vaccine of choice for outbreak control
- 9. Three Types Of OPV (mOPV), (bOPV), (tOPV) • mOPV vaccines are recommended in supplementary immunization where only WPV1 OR WPV2 is circulating • mOPV2 stockpiles are secured with WHO for any cVDPV2 after tOPV- bOPV switch • bOPV is being used in India for pulse polio immunization since 2010 • Presently tOPV is the only Oral Polio Vaccine being used for the routine immunization in OPV using countries
- 10. RISKS ASSOCIATED WITH OPV Especially in areas with low routine immunization coverage VAPP (Vaccine Associated Paralytic Polio) -Recipient VAPP - Contact VAPP VDPV (Vaccine Derived Polio Virus)
- 11. VAPP (Vaccine Associated Paralytic Polio) • Defined as those cases of AFP which have residual weakness 60 days after the onset of paralysis & from whose stool sample vaccine related poliovirus but no wild poliovirus is isolated • Caused due to loss of attenuating mutation & reversion to neurovirulance during replication of vaccine virus in the gut. • Is associated with single dose of OPV to a child or occur in a close unvaccinated or non-immune contact of vaccine recipient who is excreting the mutated virus.
- 12. VDPV(Vaccine Derived Poliovirus) • Immunized child excretes the vaccine virus for 6-8 weeks • Some of the vaccine virus gets genetically altered during replication knowns as VDPP • on very rare occasions it may revert to a form that is able to cause paralysis(VDPV) in humans • VDPV has 3 types 1. Cvdpv(circulating VDPVs): associated with sustained person-to-person transmission and circulates in community with low population immunity
- 13. 2. iVDPV(immunodeficiency related VDPVs): in immunodeficient patients who have prolonged infections after exposure to OPV. 3. aVDPV(ambiguous VDPVs): isolated from environmental sources or evidence of circulation not established
- 14. • Among these three types cVDPV causes sustained circulation. It occurs where there is low routine or supplementary immunization coverage. • cVDPV outbreaks have ability to become endemic, can spread in any under vaccinated community and can be imported to other countries. • A fully immunized population will be protected against both vaccine derived and wild polio viruses.
- 15. Birth dose of OPV • OPV at birth is not immunogenic but it enhances seroconversion of subsequent polio vaccine both OPV & IPV considerably • The first dose of OPV at a time when the infant is still protected from maternally derived antibodies may thus prevent VAPP • So it is considered necessary in countries hwere the risk of poliovirus transmission is high
- 16. INACTIVATED POLIO VACCINE • IPV was developed by Dr. Jonas Salk in 1955 • Its injectable vaccine and is available only in trivalent form • It consists of inactivated (formalin killed) strains of all three types of polioviruses(Type 1,2,3- 40D,8D,32D antigen unit). • It provides excellent humoral immunity but no mucosal intestinal immunity “SALK VACCINE”
- 17. • It is highly effective in preventing paralytic disease • In the event of infection, the antibodies produced by IPV prevent the spread of virus to CNS and protect against paralysis. • Studies in India shows that IPV given to OPV primed children boosts the mucosal intestinal immunity.
- 18. • No risk of VAPP and VDPV • IPV may contain formaldehyde, and traces of streptomycin, neomycin or polymyxin. Some formulations of IPV may contain 2- phenoxyethanol(0.5%) • IPV is freeze and heat sensitive vaccine. Stored at 2-80C in the basket of ILR
- 19. • Liquid vaccine, no reconstitution is required • Dose 0.5ml • It reduces quantity and duration of virus shedding in stool samples, which may contribute to a reduction in transmission • IPV is one of the safest vaccine in use
- 20. • IPV is not recommended for routine use in Polio-endemic countries or in developing countries at risk of poliovirus importations.
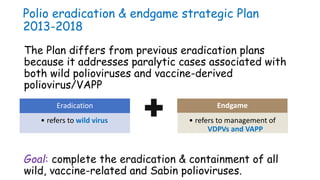
- 21. Polio eradication & endgame strategic Plan 2013-2018 The Plan differs from previous eradication plans because it addresses paralytic cases associated with both wild polioviruses and vaccine-derived poliovirus/VAPP Goal: complete the eradication & containment of all wild, vaccine-related and Sabin polioviruses. • refers to wild virus Eradication Endgame • refers to management of VDPVs and VAPP
- 22. The Plan has Four Objectives • Detect and interrupt all poliovirus transmission1 • Strengthen immunization systems, introduce inactivated polio vaccine (IPV) and withdraw oral polio vaccines (OPV)2 • Contain poliovirus and certify interruption of transmission3 • To plan how to utilize the legacy of the fight against polio4
- 23. Objective 2 of The Plan addresses the Endgame through three distinct stages Introduce • at least one dose of IPV • into routine immunization Switch • tOPV to bOPV Withdraw • of bOPV & routine OPV useBefore end 2015 2016 2019-2020 Ongoing STRENGTHENING of routine immunization services
- 24. LEGACY BUILDING • This strategic plan also aims at planning for the backbone of the polio effort to be used for delivering other health services to the worlds most vulnerable children • The assets, learning, capacities and workforces developed in the fight against polio are applied to other major public health challenges
- 25. Rational For The Introduction Of IPV • Primary purpose of introducing IPV into routine immunization is to boost population immunity against Type 2 poliovirus during & after the planned global withdrawal of OPV2 and switch from tOPV to bOPV • It will also facilitate the interruption of transmission with the use of monovalent OPV type2 in the case of outbreaks • To boost both humoral & mucosal immunity against poliovirus Type 1&3, which will also hasten the eradication of these WPVs • Mitigate the risk of emergence & transmission of cVDPV2
- 26. IPV is not replacing OPV It is a pre-requisite for tOPV to bOPV switch
- 27. Why withdraw OPV Type2 OR Why switch from tOPV to bOPV • Thus, need to remove OPV2, but need to maintain population immunity against type 2 with IPV prior to OPV2 cessation • Type 2 wild poliovirus apparently eradicated since 1999 (last case detected in Aligarh, India) • New diagnostics and experience suggest that type 2 polio vaccine causes >95% of VDPVs • Type 2 causes approximately 40% of VAPP today • Type 2 component of OPV interferes with immune response to types 1 and types 3 Risks of OPV2 far outweigh the benefits
- 28. Rational For Introducing At least One Dose Of IPV with OPV Schedule Prior to OPV2 Cessation • Combined & sequential schedule of OPV and one dose of IPV have generated high seroconvertion rate • Number of studies have shown use of both vaccines simultaneously better immune response than either vaccine alone • A single dose of IPV to children immunized previously with tOPV who are seronegative substantially improved seropositivity rates against type 2 & 3 wild polioviruses(from 91% to 100%)
- 29. Rational for introducing single dose of IPV at 14 weeks • The immune response to IPV varies based on the number of doses (higher with more doses) and the age at vaccination (higher with delayed immunization). • 3 doses: ~100% against all 3 serotypes • 2 doses: ~90% against all 3 serotypes, when given >8 weeks of age • 1 dose: ~19%-46% against Type 1, 32%-63% against Type 2, and 28%-54% against Type 3 poliovirus. • The immune response to one dose of IPV is substantially higher against Type 2 poliovirus (63%) when administered at 4 months of age compared to 6 weeks to 2 months of age (32%-39%). • Thus, SAGE recommends a single dose of IPV at 14 weeks or first contact afterwards, or with DTP3/OPV3/OPV4, in the EPI schedule
- 30. Why IPV not later than 14 weeks • The purpose of IPV is to give infants protection against type 2 VDPVs after tOPV-bOPV switch • This IPV dose will be the only protection an infant will receive against type 2 poliovirus • So vaccinating after 14 weeks will leave child unprotected for a longer period of time
- 31. • The switch is necessary because replacing tOPV with is the key to ensuring eradication of type 2 Sabin virus which in turn will reduce the risk of new cVDPV type2 outbreaks after OPV type cessation, if a cVDPV2 appears • Once the switch is made tOPV will no longer be used anywhere in the world
- 32. • Routine vaccination with IPV alone should be used only in countries with high immunization coverage(>90%) & at low risk of wild poliovirus importation and spread • When IPV is administered after a few doses of OPV, it not only enhances protection against paralytic disease but also boosts intestinal immunity even more than an additional dose of OPV would provide. • Combining IPV with OPV provides the advantages of both vaccines: Strong intestinal immunity & antibody protection against all three serotypes
- 33. AEFI IPV is one of the safest vaccine in use No serious adverse events have been reported with IPV Minor local reactions such as : redness & tenderness may occur
- 34. CONTRATINDICATIONS •Documented or known allergy to Streptomycin, Neomycin or Polymyxin B •History of allergic reaction following a previous injection of IPV
- 35. Site For Injection • Site for an injection is fixed to maintain uniformity across the country • It helps in seeking vaccination history in case of loss of MCP card • Caregiver can better recall during follow up visits • Helps ANMs to remember & safely provide multiple vaccinations
- 36. Left upper arm:- BCG, JE Right upper arm:- Measles Anterolateral aspect of right mid thigh:- IPV Anterolateral aspect of left mid thigh:- Pentavac or DPT, HepB
- 37. Four key messages for caregivers What vaccine was given & what disease it prevents What minor adverse events could occur & how to deal with them When & where to come for next visit Keep the immunization card safe & bring it along at the next visit
- 38. IPV Key Messages for Community • Children are still at risk of polio till it is not eradicated from the world • Just one dose of IPV with the third dose of OPV to your child in routine immunization at 14 weeks of age gives additional protection against polio • IPV is available free of cost at RI session site
- 39. THANK YOU!
Editor's Notes
- #7: This remarkable progress is an outcome of continuous operational improvement, adoption of best strategies, and use of appropriate vaccines. India is now guarding itself against the risk of polio resurgence from endemic countries. And reinfected countries. Also faces risk of paralysis from VDPVs in areas of low population immunity.
- #19: Liquid vaccine, no reconstitution is required.
- #26: Evidence indicate that 1 dose of ipv may reduce risk by protecting individual against paralytic polio if they r exposed to cVDPV2 or WPV2 or by enhancing the population immunity that can be achieved through use of mOPV2 in the setting of outbreak of type 2 poliovirus post OPV2 cessation bcause of proportionof popilatioon will already be immune as a result of having receiving IPV The immunity level reached after a dose of mOPV2 will be higher than the immunity level with a single dose of mOPV2 in a completely susceptible population