P block elements 1
- 6.
- 9. .
- 15. IONIZATION ENTHALPY (IE or H): Ionization enthalpy also shows irregular trends. On moving down the group, IE decreases from B to Al, but the next element Ga has slightly higher ionization enthalpy than Al due to the poor shielding of intervening d-electrons. It again decreases in and then increases in the last element .
- 16. ELECTRONEGATIVITY: Down the group the electronegativity decreases from B to Al and then increases marginally. This is due to the noticeable difference in atomic size of elements. ELEMENT ATOMIC RADIUS/pm ELECTRONEGATIVIT Y B 88 2.0 Al 143 1.5 Ga 135 1.6 In 167 1.7 Tl 170 1.8
- 17. INERT PAIR EFFECT: The phenomenon of electrons remaining paired in valance shell is called inert pair effect. It is the reluctance of the s-electron of the valance shell to take part in bonding. It occurs due to poor or ineffective shielding of the ns2 – electrons of the valance shell by intervening d and f – electrons. It increases down the group and thus, the lower elements of the group exhibit lower oxidation states.
- 18. OXIDATION STATES: B and Al show oxidation states of +3 only while Ga, In and Tl exhibit oxidation states of both +1 and +3. As we move down in the group 13, due to inert pair effect, the tendency to exhibit +3 oxidation states decreases and the tendency to attain +1 oxidation states increases. Stability of +1 oxidation state follows the order Ga<In<Tl.
- 19. F B F F + NH3 F B F F NH3 LEWIS ACIDS: Boron trifluoride is trivalent molecule of boron. The number of electrons around central boron atom in this molecule is only six. It has incomplete octet. Therefore this is electron deficient molecule and has tendency to accept lone pair of electrons to achieve stable inert gas electronic configuration. Thus it behaves as Lewis acid.
- 20. Stability in AlCl3 is formed due to the formation of dimer. Dimer: It is a molecule or molecular complex consisting of two identical molecules linked together. Al Al Cl ClCl Cl Cl Cl 10 1 79 11 8
- 21. Action of Air (oxygen): Amorphous Boron on heating in air forms B2O3 ,boron oxide. eg: 4B + 3O2 1000K 2B2O3 Boron trioxide Reactivity towards Halogens: Boron reacts with halogen to form Trihalides. eg: 2B+ 3Cl2 2BCl3 Boron trichloride
- 22. Reactivity towards Water: Pure boron does not react with water. Aluminum decomposes boiling water evolving hydrogen. eg: 2Al + 6 H2O 2Al(OH)3 + 3H2 Gallium and Indium are not attacked by pure, cold or hot water. Thallium is a little more reactive than Gallium and forms an oxide on the surface.
- 23. Boron is a nonmetal while other members are metals. Boron shows allotropy while other members do not. Amongst the elements of group 13, boron has highest M.P. and B.P. Boron forms only covalent compounds(maximum covalence of boron is 3) while other members form both ionic and covalent compounds.
- 24. Oxides and hydroxides of boron are weakly acidic, of aluminium are amphoteric while those of rest of members are most basic. Boron hydride is quite stable while hydrides of other elements are less stable. Only boron combines with active metals such as Mg to form borides while rest of the members do not. 3Mg + 2B Mg3B2 Magnesium Boride Crystalline boron is unreactive
- 25. Boron with alkali Boron dissolves with alkali , to give borates with evolution of H2 gas. 2B + 6NaOH 2Na3BO3+3H2 Reaction with acids:- 2B + 3HNO3 H3BO3+3NO2
- 32. Sum of the first three ionization enthalpies is less, as compared to Boron. this is due to the easy tendency to lose electrons It is able to form Al 3+. In the other elements, due to poor shielding effect of d and f orbitals, the nucleus holds the outer most s electrons tightly. Thus, only p bonding may be available for bonding. In all 3 elements, both +1 and +3 oxidation states are seen. The compounds in +1 state are more ionic than those in +3 state.
- 33. Aluminum forms a very thin oxide layer. With di nitrogen at high temperatures they form nitrides. It dissolves in mineral acids and aqueous alkalies and thus show amphoteric character. All the group 13 elements except thallium show reactivity towards halogens.
- 34. 2E(s) + 3O2(g) 2E2O3(s) 2E (s) + N2(g) 2EN(s) [ E = element ] 2Al(s) + 6HCl (aq) 2Al3+(aq) + 6Cl-(aq) + 3H2(g) 2E(s) + 3X2(g) 2EX3 (s)
- 36. Borax It is the most important compound of boron. It is a white crystalline solid of formula Na2B4O7⋅10H2O. Borax dissolves in water to give an alkaline solution. Na2B4O7 + 7H2O 2NaOH + 4H3BO3 Orthoboric acid On heating, borax first loses water molecules and swells up. On further heating it turns into a transparent liquid, which solidifies into glass like material known as borax bead. Na2B4O7.10H2O Na2B4O7 2NaBO2+ B2O3 sodium metaborate
- 38. Husna 11.8 ii to 11.8 iii
- 47. 450k
- 49. )
- 50. 473k
- 51. SP3 ATOMS UNDERGO HYTBRIDAZATION.THERE ARER FOUR TERMINAL B-HT NORMAL COVALENT BONDS WHICH ARE QUITE STRONG (ALSO CALLED TWO CENTRAL ELECTRONE PAIRE BOND OR TWO CENTRE TWO ELECTRONE BONDS I.E 2C-2E) AND TWO BRIDGE B….HB …B WHICH ARE DIFFERENT FROM NORMAL COVALENT BONDS AND ARE QUITE WEAK (ALSO CALLED three centre electron pair bond or three resemblance to a banana these are also called banana bond.
- 53. diethyl ether
- 57. Boron being extreamly hard refractory solid of high melting point,low density and low electrtical Conductivity, finds many applications. The main industrial application of borax and boric acid is in the manufacure of heat resistant glasses (eg. Pyrex ),glass wool and fibre glass.borax is used in flux for soldering of metals,for heat,scratch and stain resistant glazed coating to earthenwares and as consituent of medicinal soaps.an aqueous solution of orthoboric acid is generally used as a mild antiseptic.
- 58. Aluminium is a bright silvery white metal with high tensil strength.it has high eletrical and thermal conduccytivity. Aluminium forms alloy with Cu,Mg,Mn,Fe,Ni,Co. Aluminium is a chief constitiunt of silvery paints. Alums are used in purification of water. It also used in sizing of papers.it acts as septic to stop bleding.it is quite used in deying and tanning of leather.alums have good application in dehydration,decolouraton and chromatography.
- 59. Reaction with acid Aluminium dissolves in dil. or con.Hcl and dil.H2so4 SLOWLY LIBERATING H2 GAS WHILE CONC.H2SO4 GIVES SO2 GAS. 2Al+ 6HCL(dil.) + 12H2O 2[Al(H2o)]cl3 3H2
- 60. ALUMINIUM DISSOLVES IN NAOH OR KOH TO FORM META-ALUMINATE WITH THE EVOLUTION OF H2 GAS. 2Al + 2naoh+ 2H2o 2NaAlo2+ 3H2
- 61. Anam 11.11 to 11.11.6
- 62. Carbon(C), silicon(Si), germanium(Ge), tin(Sn) and lead(Pb)are the elements of group 14.
- 63. C and silicon are non metals. Ge is a metalloid whereas Pb and Sn are metal. Carbon is seventeenth most abundant element by weight in the earth crust and forms many compounds than any other elements except hydrogen.
- 64. Carbon has two stable isotopes 12C(98.9%) and 13C(1.1%). It is widely distributed in nature in free state as well as in the combined form. Carbon has third isotope as 14C. It is radioactive and used for radiocarbon dating.
- 65. In elemental form it is available in coal, graphite, diamond. Coal diamond graphite In combined form it is available as metal carbonates, hydrocarbons, and carbon dioxide gas (0.03%) in air. Carbon is versatile element in the world.
- 66. Fats, carbohydrates, proteins, vitamins, etc. and fossil fuels such as petroleum, lignite, are made up of carbon compounds, carbon is essential constituent of all living organisms.
- 67. Silicon is the second (27.7% by mass) most abundant element in the earth crust. It is present in nature as silica(SiO2) and silicates. It is a very important component of ceramics, glass and cement.
- 68. Germanium exists only in traces. Tin occurs as SnO2 (cassiterite or tinstone) and lead as galena(PbS). Ultrs pure germanium and silicon are used in making transistors and semiconductor devices.
- 70. Electronic Configuration The valence shell electronic configuration of these element is ns2np2. The inner core of the electronic configuration of element in this group differs. Hence C and Si has inert gas core. Ge and Sn have inert gas core plus 10d- electronput Pb has inert gas core plus 10d-electron and 14f-electron.
- 71. ELEMENT SYMB OL ATOMIC.NO ELECTRONIC CONFIGURATIO N Carbon C 6 [He] 2s2 2p2 Silicon Si 14 [Ne] 3s2 3p2 Germanium Ge 32 [Ar] 3d10 4s2 4p2 Tin Sn 50 [Kr] 4d10 5s2 5p2 Lead Pb 82 [Xe] 5d10 6s2 6p2
- 72. Here we are considering atomic radii, ionization enthalpy, electronegativity and physical properties. Electronic configuration of group 14 is shown in table11.7.
- 73. Atomic radii of these elements regularly increase as we move down the group primarily due to addition of new every shell at each succeeding element. The increase in atomic radii from Si onwards is however, small due to ineffective shielding of the valence electrons by the intervening d- and f- electron.
- 75. The ionization enthalpy of group 14 element is higher than the corresponding elements of group 13 due to increased nuclear charge. The influence of inner core electrons is visible . In general the ionization enthalpy decreases down the group. Small decrease in ∆H1 from Si to Ge to Sn and slight increase in ∆H1 from Sn to Pb is consequence of poor shielding effect pf intervening d and f orbitals and increase in size of atom.
- 77. Due to small size, the element of this group are slightly more electronegative than the corresponding group 13 element. The electronegativity decreases as we move down the group but not after silicon.
- 78. The electro negativity values for elements from Si to Pb are almost the same. Carbon with electronegativity of 2.5 is the most electronegative element of group 14.
- 80. The group 14 elements have four electrons in the outer most shell. All the elements show an oxidation state of +4. However as we move down the group from C to Pb, the stability of +4 oxidation state decreases while that of +2 oxidation state increases due to inert pair effect .
- 81. • Carbon also exhibits negative oxidation states. Since the sum of first four ionization enthalpies is very high, compounds in +4 oxidation states are generally covalent in nature. • In heavier elements the tendency to show +2 oxidation state increases in order Ge<Sn<Pb. • It is due to the inability of ns2 electron of valence shell to participate in bonding. • Carbon and silicon mostly show +4 oxidation state.
- 82. Germanium forms stable compounds in +4 oxidation state and very few compounds in +2 state. Tin forms divalent as well as tetravalent compounds. Sn in +2 state is reducing agent. Lead compounds are stable in +2 state. Lead in +4 oxidation states acts as oxidizing agent (PbO2). Carbon show maximum covalence as four. Other elements, because of the presence of d orbitals, show covalence more than 4
- 83. All the group 14 elements are solids. Carbon and silicon are non metals, germanium is metalloid, where as tin and lead are soft metals with low melting points.
- 84. The m.p and b.p of group 14 elements are higher than those of corresponding elements of group 13. This is due to strong interacting binding forces in their solids as well as in liquid states. Down the group, there is regular decrease in m.p and b.p as the size of atoms increases and interatomic forces of attraction decreases. Hence silicon is hard while lead is a soft metal.
- 94. Allotropes are different physical forms of the same element which have same chemical properties but different physical properties, and the phenomenon is known as Allotropy.
- 95. Carbon can bond with itself in at least three different ways giving us 3 different materials Diamond Graphite Buckminster-fullerenes
- 97. A diamond is something known as an allotrope. An extremely hard, highly refractive crystalline formof c arbon that is usually colourless and is used as gemstone and in abrasives, cutting tools, and other applications.
- 98. Carbon has an electronic arrangement of 2,4. In diamond, each carbon shares electrons with four other carbon atoms - forming four single bonds. In the diagram some carbon atoms only seem to be forming two bonds (or even one bond), but that's not really the case. We are only showing a small bit of the whole structure.
- 99. High melting point due to strong directional covalent bonds (3550 C). Extremely hard because it is difficult to break atoms apart or move them in relation to one another. No electrical conductivity because electrons are localized in specific bonds. Insoluble in polar and non-polar solvents because molecular bonds are stronger than any intermolecular forces.
- 100. A soft crystalline allotrope of carbon, composed of graphene layers, having a steel-gray to Black metallic luster and a greasy feel, used in lead pencils, lubricants, paints.
- 101. Each carbon atom is only covalently bonded to three other carbon atoms, rather than to four as in diamond. Graphite contains layers of carbon atoms. Graphite has a layer structure which is quite difficult to draw convincingly in three dimensions. The diagram below shows the arrangement of the atoms in each layer, and the way the layers are spaced.
- 102. Has a high melting point, similar to that of diamond. Has a soft, slippery feel, and is used in pencils and as a dry lubricant for things like locks. Has a lower density than diamond. Conducts electricity. The delocalised electrons are free to move throughout the sheets. Is insoluble in water and organic solvents.
- 103. A fullerene is a pure carbon molecule composed of at least 60 atoms of carbon. Because a fullerene takes a shape similar to a soccer ball or a geodesic dome, it is sometimes referred to as a buckyball .
- 104. They consist of hexagonal rings of carbon atoms (like in graphite or graphene) and alternating pentagonal carbon rings to allow curvature of the surface The carbon-carbon bonds in Buckminster Fullerene C60 form a pattern like a soccer ball and this fullerene is a brownish-reddish- magenta colour when dissolved in organic solvents.
- 105. The molecule can act as a semiconductor, conductor and superconductor under specific conditions Fullerenes can display the photochromic effect, which is a change in light transmission based on intensity Is essentially insoluble in polar solvents, sparingly soluble in alkanes Fullerenes are relatively safe and inert, and yet have properties that allow the substance to create active derivatives
- 106. Direct combination of carbon in limited supply of air or oxygen gives carbon monoxide 2C(s)+O2 2CO(g) C O Carbon monoxide is both short-lived in atmosphere and spatially variable in concentration Chiefly it is a product of volcanic activity but also man-made fires, burning of fossil fuels also contributes to carbon monoxide production
- 108. Chemical formula- CO Appearance- Colorless Odor- Odorless Solubility- soluble in chloroform, acetic acid, ethyl acetate, ethanol, ammonium, hydroxide, benzene A powerful reducing agent and reduces almost all the metal oxides other than alkali and alkaline earth metals and few transitional elements It is used in extraction of many metals from their oxides
- 109. Fe2 O3 +3CO 2Fe(s) +3CO2(g) CuO(s) + CO(g) Cu(s) + co2(g) ZnO(s) + CO(g) Zn(s) + CO2(g) Iron oxide + Iron + carbon dioxideCarbon monoxide Copper oxide + Carbon monoxid e copper+ carbon dioxide Zinc oxide + Carbon monoxide Zinc + Carbon dioxide
- 110. Hemoglobin carries oxygen and carbon dioxide Hemoglobi n Red Blood Cell Carbon monoxide binds very tightly to hemoglobin O2 and Co2 can no longer be
- 111. ) ∆ ∆ 2) It is prepared in the laboratory by the action of dilute HCl on calcium carbonate. ∆ KHATIJA 11.17.2 TILL SILCON DI OXIDE
- 112. 3)On commercial scale, it is obtained by heating lime stone. 4) It is valuable byproduct in the manufacture of ethyl alcohol by fermentation of glucose and fructose
- 114. It is a colourless and odourless gas .Its low solubility in water makes it of great biochemical and geo chemical importance. With water it forms carbonic acid which is a dibasic acid and disassociates in two steps H2CO3/HCO3 - buffer system helps to maintain pH of blood between 7.26 to 7.42.
- 115. sunlight 453-473K 220 atm Ammonium carbonate
- 123. ―Si―O―Si―O―Si―O―Si― O O O O ―Si―O―Si―O―Si―O―Si― O O O O ―Si―O―Si―O―Si―O―Si― | | | | | | | | | | | | | | | | | | | | | | | | FIG: 11.11 STRUCTURE OF SiO2
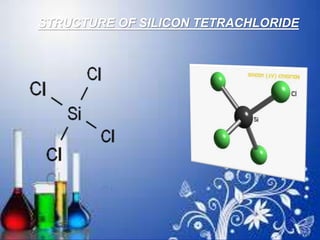
- 125. SILICON TETRACHLORIDE SiCl4 Silicon tetrachloride is the inorganic compound with the formula SiCl4. It is a colourless volatile liquid that fumes in air. It is used to produce high purity silicon and silica for commercial applications
- 126. It is prepared by action of chlorine on silicon Si + 2Cl2 SiCl4 This tetrahalide is covalent in nature and possess tetrahedral geometry. The tetra chloride of carbon i.e.CCl4 is not hydrolysed by water but SiCl4 gets easily hydrolysed. CCl4 +H2O No reaction SiCl4 + 4H2O Si(OH)4 + 4HCl orthosilicic acid
- 127. EXPLANATION Silicon and carbon both are the members of 14th group. But Silicon tetrachloride reacts with water while carbon tetrachloride does not . this is due to the fact that the carbon does not have d - orbitals to accept lone pair of electron from water while silicon has vacant d - orbitals to accept lone pair of electron from water.
- 128. SILICONES Silicones are polymers that include any inert, synthetic compound made up of repeating units of siloxane, which is a chain of alternating silicon atoms and oxygen atoms, frequently combined with carbon and/or hydrogen. They are typically heat- resistant and rubber-like, and are used in sealants, adhesives, lubricants, medicine, cooking utensils, and thermal and electrical insulation. Some common forms include silicone oil, silicone grease, silicone rubber, silicone resin, and silicone caulk.
- 129. FORMATION OF SILICONES Effective lubrication is provided by an interfacial monomolecular film of coadsorbed dimethylsilicone and fatty acid molecules. This film continues to maintain low friction and wear with increasing temperature due to the reaction of the acid with the metal surfaces and the subsequent formation of the high-melting-point copper soap. Electrical resistance measurements show this to be due to the formation of a thin non-conducting interfacial film, which provides protection for the sliding surfaces up to temperatures in excess of 150°C.
- 130. Soup ladle and pasta ladle are made up of silicones A silicone food steamer to be placed inside a pot of boiling water Ice cube trays made of silicon
- 131. SILICATES A silicate is a compound containing an anionic silicon compound. The great majority of silicates are oxides, but hexafluorosilicate ([SiF6]2−) and other anions are also included. "Orthosilicate" is the anion SiO4 4− or its compounds. Related to orthosilicate are families of anions (and their compounds) with the formula [SiO2+n]2n−. Important members are the cyclic and single chain silicates {[SiO3]2−}n and the sheet-forming silicates {[SiO2.5]−}n. Silicates comprise the majority of Earth's crust, as well as the other terrestrial planets, rocky moons, and asteroids.
- 133. The main applications were in detergents, paper, water treatment, and construction materials
- 134. STRUCTURE OF SILICON TETRACHLORIDE
- 135. Silicates Feldspar -NaAlSl3o8 Mica- KH2Al3(Sio4) or KAl3Si3O(OH) Zeolite – Na2O.Al2O3.XSiO2.YH2O Structure of silicate:-silicates are is sp3of SiO4 tetrahedral units silicon is Sp3 hybridized and surrounded by 4 oxygen atoms When all four corners are shared with other tetrahedral units, a three dimensional network is formed and –ve charge on silicate structure is neutralized by +vely charge metal ions.
- 136. ZEOLITES Zeolites are microporous, aluminosilicate minerals commonly used as commercial adsorbents and catalysts.The term zeolite was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that upon rapidly heating the material stilbite, it produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek ζέω (zéō), meaning "to boil" and λίθος (líthos), meaning "stone". Zeolites occur naturally but are also produced industrially on a large scale. As of July 2015, 229 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known.[
- 137. Zeolites are widely used as ion-exchange beds in domestic and commercial water purification, softening, and other applications. In chemistry, zeolites are used to separate molecules (only molecules of certain sizes and shapes can pass through), and as traps for molecules so they can be analyzed.


































![ 2E(s) + 3O2(g) 2E2O3(s)
2E (s) + N2(g) 2EN(s)
[ E = element ]
2Al(s) + 6HCl (aq) 2Al3+(aq) + 6Cl-(aq) +
3H2(g)
2E(s) + 3X2(g) 2EX3 (s)](https://image.slidesharecdn.com/pblockelements1-160928134343/85/P-block-elements-1-34-320.jpg)























![ Reaction with acid
Aluminium dissolves in dil. or con.Hcl
and dil.H2so4 SLOWLY LIBERATING H2 GAS
WHILE CONC.H2SO4 GIVES SO2 GAS.
2Al+ 6HCL(dil.) + 12H2O
2[Al(H2o)]cl3 3H2](https://image.slidesharecdn.com/pblockelements1-160928134343/85/P-block-elements-1-59-320.jpg)











![ELEMENT
SYMB
OL ATOMIC.NO
ELECTRONIC
CONFIGURATIO
N
Carbon C 6 [He] 2s2 2p2
Silicon
Si
14 [Ne] 3s2 3p2
Germanium
Ge
32 [Ar] 3d10 4s2 4p2
Tin Sn 50 [Kr] 4d10 5s2 5p2
Lead Pb 82 [Xe] 5d10 6s2 6p2](https://image.slidesharecdn.com/pblockelements1-160928134343/85/P-block-elements-1-71-320.jpg)



























































![SILICATES
A silicate is a compound containing an
anionic silicon compound. The great
majority of silicates are oxides, but
hexafluorosilicate ([SiF6]2−) and other
anions are also included.
"Orthosilicate" is the anion SiO4
4− or its
compounds. Related to orthosilicate are
families of anions (and their
compounds) with the formula
[SiO2+n]2n−. Important members are the
cyclic and single chain silicates
{[SiO3]2−}n and the sheet-forming
silicates {[SiO2.5]−}n.
Silicates comprise the majority of
Earth's crust, as well as the other
terrestrial planets, rocky moons, and
asteroids.](https://image.slidesharecdn.com/pblockelements1-160928134343/85/P-block-elements-1-131-320.jpg)