Phase solubility analysis and pH solubility profile
- 1. PHASE SOLUBILITY ANALYSIS AND PH SOLUBILITY PROFILE Presented By: Mohit Mahesh Angolkar I M.Pharm (Industrial Pharmacy) JSS College of Pharmacy, Mysore Subject: Pharmaceutical Formulation Development (MIP 102T) Guided By: Dr. D. V. Gowda Professor and Head Department of Pharmaceutics JSS College of Pharmacy, Mysore
- 2. SOLUBILITY ➤ Solubility may be defined as the maximum concentration of a substance that may be completely dissolved in a given solvent at a given temperature and pressure. ➤ The USP/NF generally expresses the solubility in terms of the volume of solvent required to dissolve 1 gram of the drug at a specified temperature. 2
- 3. ➤ In Qualitative terms: Solubility is defined as, “the spontaneous interactions of two or more substances to form a homogenous molecular dispersion.” ➤ In Quantitative terms: Solubility is defined as, “the concentration of a solute in a saturated solution at a constant temperature.” 3
- 4. ➤ Solubility can be categorised into: Unbuffered solubility: Describes the solubility of a solution saturated with a compound at the terminal pH of the solution. Buffered solubility: Describes the solubility at a specific pH. Intrinsic solubility: Describes the solubility of an ionisable compound in its natural form. 4
- 5. IMPORTANCE OF SOLUBILITY ➤ Therapeutic effectiveness of a drug depends upon the bioavailability and ultimately upon the solubility of drug molecules. ➤ Currently only 8% of new drug candidates have both high solubility and permeability. ➤ Nearby 40% of the new chemical entities currently being discovered are poorly water soluble. ➤ Low aqueous solubility is the major problem encountered with formulation development of new chemical entities. ➤ Any drug to be absorbed must be present in the form of an aqueous solution at the site of absorption. 5
- 6. NEED FOR SOLUBILITY ENHANCEMENT ➤ For a drug to enter the systemic circulation and exert a therapeutic effect, it must first be in solution. ➤ Hence, two areas of pharmaceutical research that focus on improving the oral bioavailability of active agents include; enhancing of solubility and dissolution rate of poorly water soluble drugs. ➤ There are variety of new drugs & derivatives are available. But 40% of lipophilic drugs candidates fail to reach market due to poor bioavailability, even though these drugs might exhibit pharmacodynamic activities. ➤ The lipophilic drug that reaches market requires a high dose to attain proper pharmacological action. ➤ The basic aim of Further formulation & development is to make that drug available at proper site of action within optimum dose. 6
- 7. FACTORS INFLUENCING SOLUBILITY ➤ Temperature : Around 95% of solid solutes, the solubility increases with temperature, but gaseous solutes exhibit more complex behaviour. As the temperature is raised gases usually become less soluble in water, but more soluble in organic solvents. 7
- 8. ➤ Effect of Pressure: Liquids and solids exhibit practically no change of solubility with changes in pressure. Gases as might be expected increase in solubility with an increase in pressure. Henry's Law states that: The solubility of a gas in a liquid is directly proportional to the pressure of that gas above the surface of the solution. If the pressure is increased, the gas molecules are "forced" into the solution. ➤ Effect of Molecular Size: Molecular Size will affect the solubility. The larger the molecule or the higher its molecular weight the less soluble the substance will be. Larger molecules are more difficult to surround with solvent molecules in order to solvate the substance. 8
- 9. ➤ E ff ect of Particle size: The particle size and surface area of a drug exposed to a medium can a ff ect actual solubility of drug. Small increase in solubility can be accomplished by particle size reduction. ➤ E ff ect of pH: The e ff ect of pH on solubility is critical in the formulation process. Adjustment of pH usually has little e ff ect on the solubility of substances other than electrolytes. In many cases, it is desirable to use cosolvents or other techniques such as complexation, micronization, or solid dispersion to improve aqueous solubility. As the pH of the solution increases, the quantity of drug in solution increases . HA↔H + A + - Ka 9
- 11. PHASE SOLUBILITY ANALYSIS ➤ Phase solubility analysis is a simple and elegant technique whereby absolute purity of a crystalline material can be determined. ➤ Phase solubility analysis is the quantitative determination of the purity of a substance through the application of precise solubility measurements. ➤ At a given temperature, a definite amount of a pure substance is soluble in a definite quantity of solvent. ➤ The resulting solution is saturated in respect of the particular substance. ➤ But the solution remains unsaturated in respect of other substances even though such substances may be closely related in chemical structure and physical properties to the particular substance being tested. 11
- 12. ➤ Constancy of solubility indicates that a material is pure or free from foreign substances. ➤ Phase solubility analysis is a nondestructive physical method for the quantitative determination of the composition of substances and is applicable to all classes and species of molecules. ➤ It requires simple, inexpensive equipment commonly available in laboratories. ➤ By this technique, one may determine simultaneously the total amount and number of impurities in a substance and the solubilities of these impurities as well as that of the main substance. ➤ Applicable- Crystalline solids that form stable solution. ➤ Not applicable- solid solutions. 12
- 13. ➤ It is based on the Gibbs Phase Rule. ➤ Gibbs' phase rule describes the possible number of degree of freedom (F) in a closed system at equilibrium, in terms of the number of separate phases (P) and the number of chemical components (C) in the system. ➤ It was deduced from thermodynamic principles by Josiah Willard Gibbs in the 1870s. ➤ Mathematically, this rule, F = C+ 2—P, relates the number of components, C, the degrees of freedom (temperature, pressure, and concentration), F , and the phases, P. F = C+ 2—P ….. (1) 13
- 14. ➤ A phase is a homogeneous, physically distinct, and mechanically separable portion of matter. ➤ In phase solubility analysis, temperature and pressure are maintained constant and, therefore, there is just one degree of freedom, the concentration. i.e F° = F — 2 …. (2) ➤ Substituting equation (2) in Gibbs equation, we obtain, i.e C = P + F° …. (3) 14
- 15. PHASE SOLUBILITY DIAGRAM ➤ When a pure solid is brought into contact with a liquid in which it is soluble, a certain amount of it passes into solution and this process continues until the concentration reaches a definite value independent of the amount of solid present. ➤ A condition of equilibrium is established between the solid and the solution; i.e., the solution becomes saturated with the solute. For a pure solid in solution, one phase is present (two components) F° = 1. ➤ For a pure solid in contact with its saturated solution at equilibrium (BC), two phases are present, solid and solution; there can be no variation in concentration, F° = 0. 15
- 16. PHASE SOLUBILITY DIAGRAM System composition mg/g 16
- 17. STANDARD SOLUBILITY METHOD 17 Mixing of material with solvent Establishment of equilibrium Separation of solid phase from solution Determination of concentration of material dissolved per unit of solvent Determination of weight of material unit of solvent Plotting graph, Extrapolation and calculation
- 18. SOLVENTS ➤ The proper solvent or solvent system has following characteristics: Sufficient volatility Boiling points between 60°C and 150 °C. Does not adversely affect the test, compound. Has known purity and composition. The test compound has solubility of about 10-20 mg/ml in the solvent or solvent system. So the solvents that solubilize the drug substance at concentration greater than 20 mg/ml can be used. 18
- 19. ➤ Solvents Commonly Used in Phase Solubility Analysis: Ethyl acetate Benzene Dioxane Dimethylformamide (DMF) Hexane Cyclohexane Water Acetone and aqueous mixtures Ethanol and aqueous mixtures Methanol and aqueous mixtures Isopropanol and aqueous mixtures n-Propanol and aqueous mixtures Pyridine and aqueous mixtures Chloroform and alcoholic mixtures 19
- 20. APPARATUS ➤ Constant Temperature Bath Use a constant temperature bath that is capable of maintaining the temperature within ± 0.1̊ and that is equipped with horizontal shaft capable of rotating at approximately 25 rpm. The shaft is equipped with clamps to hold the Ampoules. Alternatively, the bath may contain suitable vibrator, capable of agitating the ampoules at 100 to 120 vibrations per second, and equipped with the shaft and suitable clamps to hold the ampoules. 20
- 21. ➤ Ampoule and Solubility fl ask: Ampoule (left) and solubility flask (right) used in phase solubility analysis 21
- 22. PROCEDURE ➤ System composition: Increased amount of sample 5ml solvent Cool in dry acetone Double jet air gas burner Weigh all ampoules 22
- 23. ➤ Calculate the system composition, in mg per g, for each ampoule by the formula:- 1000(W2 - W1)/(W3 - W2) In which, W1 = Weight of empty ampoule W2 = Weight of ampoule with test substance W3 = Weight of ampoule with test substance and solvent 23
- 24. ➤ Equilibration: The time required for equilibration varies with the substance, the method of mixing (rotation or vibration), and the temperature. 24 Determination of Equilibration One ampoule - next to last in the series Super saturated solution Solubility of test species 400 C
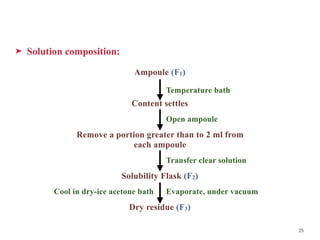
- 25. ➤ Solution composition: Ampoule (F1) Content settles Remove a portion greater than to 2 ml from each ampoule Solubility Flask (F2) Dry residue (F3) Temperature bath 25 Open ampoule Transfer clear solution Evaporate, under vacuum Cool in dry-ice acetone bath
- 26. ➤ Calculate the solution composition, in mg per g, by the formula: Csolution (mg/g) =1000 x (F3-F1)/F2-F3) In which F1 = weight of the flask plus residue, F2 = weight of the solubility flask, F3 = weight of the flask plus solution 26
- 27. ➤ Calculation: Plot the ratio of the weight of the dissolved materials per weight of solvent (Y axis or solution composition) against the ratio of the total weight of material per weight of solvent (X axis or system composition). The points for those containers which represent a true solution should fall on a straight line (AB) with a slope approaching 1, passing through the origin. The points corresponding to saturated solutions should fall on another straight line (BC), the slope, S, of which represents the fraction of impurity or impurities present in the test substance. 27
- 28. ➤ The slope may be calculated by the formula, S= (Y2 -Y1 )/(X2 -X1 ) in which, Y2 and Y1 represent solution compositions X2and X1 represent the respective system compositions. ➤ Calculate the per cent purity of the test substance by the formula, % PURITY = 100 — 100S ➤ The solubility of the main component is obtained by extending the solubility line (BC) through the Y-axis. ➤ The extrapolated solubility, obtained in mg per g, should be constant for a given compound. 28
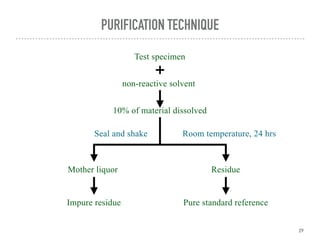
- 29. PURIFICATION TECHNIQUE Test specimen non-reactive solvent 10% of material dissolved Seal and shake 29 Room temperature, 24 hrs Mother liquor Residue Pure standard reference Impure residue
- 30. APPLICATIONS ➤ Employed routinely for purity determination, particularly in the pharmaceutical industry. ➤ Purity of a substance can be determined even in absence of an assay. ➤ Many proteins are purified by this technique. ➤ The solubility technique can be modified to study the extent of non- covalent interaction between two solutes. ➤ This technique is used to determine the solubility of steroids in mixtures of organic solvents. 30
- 32. PH SOLUBILITY PROFILE ➤ pH is one of the primary in fl uences on the solubility of most drugs that contain ionisable groups. Solubility of acidic, basic and amphoteric drugs as a function of pH. 32
- 33. ➤ Acidic drugs: Acidic drugs, such as the non-steroidal anti-inflammatory agents, are less soluble in acidic solutions than in alkaline solutions. The equation relating the solubility, S, of an acidic drug to the pH of the solution is: where So is the solubility of the undissociated form of the drug. ➤ Basic drugs: Basic drugs such as ranitidine are more soluble in acidic solutions where the ionised form of the drug is predominant. The equation relating the solubility, S, of a basic drug to the pH of the solution is: 33
- 34. ➤ Amphoteric drugs: Amphoteric drugs such as the sulfonamides and tetracyclines display both basic and acidic characteristics. The zwitter ion has the lowest solubility, So, and the variation of solubility with pH is given by: at pH values below the isoelectric point at pH values above the isoelectric point 34
- 35. ➤ Many drugs are weak organic acids (for eg, acetylsalicylic acid) or weak organic bases (for eg, procaine) or their salts (for eg, ephedrine hydrochloride). ➤ A weak acid or base is only slightly ionised in solution, unlike a strong acid or base, which is completely ionised. ➤ The exceptions to this general statement are the non- electrolytes, such as the steroids, and the quaternary ammonium compounds, which are completely ionised at all pH values and in this respect behave as strong electrolytes. ➤ The extent of ionisation of a drug has an important effect on its absorption, distribution and elimination. 35
- 36. IONISATION OF DRUGS IN SOLUTION ➤ Ionisation of weakly acidic drugs and their salts: If the weak acid is represented by HA, its ionisation in water may be represented by the equilibrium: HA + H2O!A-– + H3O++ The equilibrium constant, Ka, is referred to as the ionisation constant, dissociation constant or acidity constant and is given by: K= [H3O+][A-] /[HA] The negative logarithm of Kais referred to as pKa, i.e. pKa = –logKa. 36
- 37. When the pH of an aqueous solution of the weakly acidic drug approaches to within 2 pH units of the pKa there is a very pronounced change in the ionisation of that drug. The percentage ionisation at a given pH can be calculated from: Salts of weak acids are essentially completely ionised in solution, for example when sodium salicylate is dissolved in water, it ionises almost entirely into the conjugate base of salicylic acid. 37
- 38. ➤ Ionisation of weakly basic drugs and their salts: If the weak acid is represented by B, its ionisation in water may be represented by the equilibrium: B + H2O!BH+ + OH– The equilibrium constant, Kb, is referred to as the ionisation constant, dissociation constant or basicity constant and is given by: The negative logarithm of Kb is referred to as pKb, i.e. pKb= –logKb. 38
- 39. ➤ The percentage ionisation at a given pH can be calculated from: ➤ Salts of weak bases are essentially completely ionised in solution; for example, ephedrine hydrochloride exists in aqueous solution in the form of the conjugate acid of the weak base. 39
- 40. ➤ Ionisation of amphoteric drugs: These can function as either weak acids or weak bases in aqueous solution depending on the pH and have pKavalues corresponding to the ionisation of each group. If pK of the acidic group, pKa acidic , is higher than that of the basic group, pKa basic , they a are referred to as ordinary ampholytes and exist in solution as a cation, an unionised form, and an anion depending on the pH of the solution. If pKa acidic < pKa basic they are referred to as zwitter ionic ampholytes and exist in solution as a cation, a zwitter ion (having both positive and negative charges), and an anion depending on the pH of the solution. 40
- 41. REFERENCES ➤ Ketan T. Savjani, Anuradha K. Gajjar, Jignasa K. Savjani. Drug Solubility: Importance And Enhancement Techniques. Review Article. ISRN Pharmaceutics; volume 2012. ➤ Sandeep Kumar, Preetam Singh. Various Techniques For Solubility Enhancement: An Overview. The Pharma Innovation; 2016: 5(1). 23-28. ➤ William J. Mader & Takeru Higuchi (1970) Phase Solubility Analysis, C R C Critical Reviews in Analytical Chemistry, 1:2, 193-215. ➤ Patrick J. Sinko. Martin’s Physical Pharmacy And Pharmaceutical Sciences. Solubility And Distribution Phenomenon. 7Th Edition. 186-202. ➤ Jadhav P. B., Pandey P.S. Phase Solubility Analysis: A Technique Of Purity Determination. World Research Journal Of Pharmaceutical Research, Volume 1, Issue 1, 2013. 05-11. ➤ D. C. Garrett, C. A. Johnson, R. E. King. Phase Solubility Analysis: An Evaluation Of The Technique. ➤ David Attwood, Alexander Florence. Fasttrack Physical Pharmacy. Solubility And Solutions Properties Of Drugs. 11-26.
- 42. THANK YOU

































![IONISATION OF DRUGS IN SOLUTION
➤ Ionisation of weakly acidic drugs and their salts:
If the weak acid is represented by HA, its ionisation in water may be
represented by the equilibrium:
HA + H2O!A-–
+ H3O++
The equilibrium constant, Ka, is referred to as the ionisation constant,
dissociation constant or acidity constant and is given by:
K= [H3O+][A-] /[HA]
The negative logarithm of Kais referred to as pKa, i.e. pKa = –logKa.
36](https://image.slidesharecdn.com/phasesolubilityanalysisandphsolubilityprofile-211214072542/85/Phase-solubility-analysis-and-pH-solubility-profile-36-320.jpg)





