Process validation
- 1. 6th CPH assessment training workshop May 2014 Process validation
- 2. Talk points Objectives of review of quality(CMC) data- reminder Process validation, definition and current approaches Role of dossier assessment in process validation Risk assessment as part of process validation Validation scheme: Monitoring and Sampling Specific topics: Blend uniformity and validation of compression step Process validation: other dosage forms Process validation commitment Retrospective validation Summary: How to review protocol and report
- 3. Reminder Objectives of assessment of quality part To provide the highest assurance that all production batches (unit doses) will be consistently efficacious as the clinical batch(es) To reduce risk to safety via the highest assurance of acceptable and consistent quality of the product and its components Process validation
- 4. Process validation The collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality products. (FDA) Documented evidence which provides a high degree of assurance that a specific process will consistently result in a product that meets predetermined specifications and quality characteristics. (WHO) The documented evidence that the process, operated within established parameters, can perform effectively and reproducibly to produce a medicinal product meeting its predetermined specifications and quality attributes.(EMA)
- 5. Process validation Traditional vs new paradigm Post approval changes/chan ge controls/risk analysis Development- Basic Process validation- 3 batches Pilot batch manufacturing Enhanced- Development and process qualification Control Strategy Continuous and extensive monitoring of CQAs and CPPs for each production batch ICH Q9 and Q10 ICH Q8, QbD
- 6. Latest guidelines FDA, January 2011 WHO, Revised Annex 7 of WHO GMP guide (draft for comment) EMA, February 2014 Continuous process verification (CPV) Continuous process verification (CPV) Alternative approaches: -Traditional approach -Continuous process verification -Hybrid approach Process design and Initial validation (process qualification- PPQ) are initial phases of CPV Process design and initial validation (initial process verification) are initial phases of CPV CPV protocol to be supported by extensive development information and lab or pilot scale data. Executed on each production batch No mention of number of batches for initial process performance qualification/validation (rather must be justified based on overall product and process understanding) Mentions data on at least three pilot or production batches collected as part of process design Number of batches specified for traditional approach - minimum of three production batches unless other wise justified
- 7. Types of process validation and dossier requirements Prospective validation Concurrent validation Retrospective validation Protocol reviewed and accepted, Product PQD; OR Protocol executed before submission or PQ Protocol reviewed and accepted, Product PQD Protocol does not need to be submitted Execute and finalize process validation on the first three production batches Execute and finalize process validation on the first three production batches Prepare product quality review report on already manufactured production batches Commercial batches to be released only after satisfactorily conclusion of process validation on three batches Each validation batch can be validated and released. Applicable for low demand products (such as NTDs, orphan drugs or other seasonal products) Applicable for submissions meeting criteria for established products as described in Annex 4, TRS 970
- 8. Process validation- Role of assessment Design qualification Operational qualification Performance qualification Process validation GMP Dossier
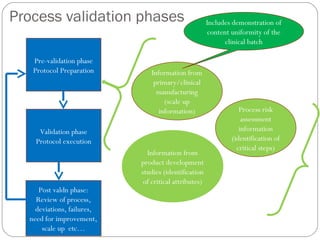
- 9. Process validation phases Pre-validation phase Protocol Preparation Information from product development studies (identification of critical attributes) Information from primary/clinical manufacturing (scale up information) Process risk assessment information (identification of critical steps) Validation phase Protocol execution Post valdn phase: Review of process, deviations, failures, need for improvement, scale up etc… Includes demonstration of content uniformity of the clinical batch
- 10. Risk assessment Part of process development and protocol preparation Risk matrix- usually as part of process development Critical quality attributes (CQA) vs processing stages, e.g. dissolution vs granulation CQA vs critical process parameters, e.g., dissolution vs kneading time Failure mode analysis- usually as part of process validation To identify critical attributes, processes and parameters Informed validation To establish control strategy
- 11. Example: risk matrix for low dose capsule (CQA vs process stages) Sifting/sizing blending lubrication Capsule filling Assay Low Medium Medium Medium Content uniformity High High High High Dissolution Low Low High Low Stability Low Low Low Low
- 12. Process steps to be validated All steps that are generally considered critical (medium and high risk steps) should be monitored/scrutinized by summarizing actual process parameters applied and observations recorded e.g. sifting stage, wet and dry granulation stages observations serve as feedback for future refinement of process parameters In addition, where feasible, sampling and testing should be performed e.g. drying, mixing steps, compression, filling results measure effectiveness and consistency of the immediate as well as preceding steps- e.g. final blend characteristics are mainly shaped by wet/dry granulation process
- 13. Validation scheme- example Processing steps Critical parameters Validation scheme Dispensing Weight checks Monitored Sifting Mesh size Monitored Wet Granulation and drying Amount and addition rate of granulating agent, mixing speed, time, as well as sequence of events Monitored, Drying uniformity to be tested Dry Granulation Slugging /compaction parameters Monitored only or Monitored and sampled? Blending mixing speed, time Monitored; Blend uniformity to be established Lubrication mixing speed, time Monitored; Blend uniformity from mixer and bulk container Compression Initial set up parameters, speed, applied pressure, Monitored; Several samples to be sampled and tested for IPQC parameters Fluidized bed coating Spray rate, inlet and product temp, etc… Monitored; appearance, weight gain and full testing Primary packaging, protocol requested on case by case basis Sealing temperature, speed Monitored; leak test
- 14. Monitoring- Example: Compaction Any comment vis à vis the difference between BMR set range and actual applied inputs? BMR Set parameters Batch 1 Batch 2 Batch 3 e.g. of parameter s Cycle 1 Cycle 2 Cycle 1 Cycle 2 Cycle 1 Cycle 2 Roller speed (RPM) 8-15 10 10 10 10 10 10 Roller pressure (Bars) 40-60 41-42 42-43 41-43 41-42 41-42 41-43 Vertical feed screw (RPM) 50-100 75 75 75 75 75 75 Horizontal feed screw (RPM) 10-20 15 15 15 15 15 15
- 15. Example: Monitoring and sampling: Drying Monitoring Set parameter Observation Batch X Batch Y Batch Z Inlet temperature 60+/-10o C 62-65 52-63 52-60 Outlet temp 29-44 31-47 28-36 Total drying time (min) (for information) 65 65 80 Sampling and testing Spec Batch X Batch Y Batch Z Location 1 0.75-2.25% 1.54 1.53 1.70 Location 2 1.94 2.01 1.80 Location 3 2.03 1.30 2.05 Location 4 1.89 1.87 2.20
- 16. Blend uniformity Early check for content uniformity of the final dosage form Uniform blend with good flow and compressibility characteristics Compression with optimum conditions Tablets meeting criteria for uniformity of dosage units Note: Blend uniformity is a routine test for low dose products (i.e. active load <=5% or 5mg)
- 17. Blend uniformity- Sampling location and method Sampling location -usually predetermined as part of qualification of the mixer (i.e. mostly GMP issue) But, in the dossier, we at least check if periphery, center positions and various other positions are considered Samples from each location are usually taken in triplicate Samples should also be taken from the blend container- to evaluate impact of transfer important for low dose products and particularly for DC processed blend Sampling should be done consistently and in away that does not disturb the bulk blend state – such aspects (e.g. type of sampling thief used) are better addressed at the time of inspection
- 18. Blend uniformity- Sample size What is an acceptable amount for samples taken at each location? Normally 1-3 time of the FPP unit dose weight
- 19. Blend uniformity- acceptance criteria Commonly used criteria Individual assays: 90.0-110.0% of label claim, RSD NMT 5.0% Less common Individual assays:90.0-110.0% of the mean value, RSD NMT 5.0% In this case, setting mean = 95.0-105.0% of the label claim appears reasonable Rarely (in case of very low dose products) Individual assays: 85.0-105.0% of the label claim/mean value, RSD: NMT 5.0% May be acceptable provided that uniformity of dosage units is satisfactorily demonstrated on tablets/capsules manufactured from blend lot with close to limit blend uniformity results
- 20. Sampling and testing plan- Lubrication- example missing parameter?Do you agree with the acceptance criteria? Sample location Sample size Sample analysed Tests Acceptance limits Lubrication 10 position from Octagonal blender and blend container 850-2550mg in triplicate 10 Individual samples Blend uniformity Mean: 95.0- 105.0%, individual: 90-110%, RSD: NMT 5% Samples from top, middle and bottom 50gm Composite samples Complete analysis as per routine blend spec As per blend spec Particle size distribution, bulk and tapped density For information What are the minimum tests we expect to see in blend spec? Acceptable?
- 21. Compression Good compression outcome is a measure of (it depends on):- Granule/powder mix properties bulk and tapped density-granulation particle size and particle size distribution-granulation moisture content- drying extent of lubrication- lubrication time Machine and tooling attributes appropriate selection and adequate lubrication of punches and dye machine speed applied compression pressure
- 22. Compression – Sampling frequency and size depends on the length of the run time/ batch size we expect frequent sampling than the normal IPQC frequency the number of tablets/capsules taken should be greater than those taken during a normal IPQC sampling
- 23. Compression- Challenge studies Certain variations in compression speed and hardness than the target set points may happen what would be the impact of such variations? speed affects dwell time- which intern affects several tablet parameters (thickness, hardness, as well as weight variation) Therefore, robustness should be demonstrated C. Morten, PIAT programme, University of Manchester
- 24. Extensive sampling- example (there are several other approaches) IPQC testing schedule Normal production batch Validation batches 48 station machine, batch size of 170,000 tabs, target speed 25rpm Group weight and appearance, every 30 minutes; others every 1 hour (at least 3 times) About 300 tablets About 300 tablets All in process parameters at start, middle and end of compression (different hopper fill levels) - About 360 tablets Additional samples at high, low speed; at high and low hardness levels - About 480 samples Total number of tablets sampled 300 tablets 1140 tablets
- 25. How to demonstrate consistency? 3sigma process e.g. 4 sigma process
- 26. Process validation-oral solutions Validation focuses on mixing time and conditions to clear solution, if deemed relevant bulk liquids: pH, specific gravity, clarity of solutions; assay filling process filled units:-Volume/Wt variation and as per FPP specs Protocol with commitment is acceptable at the time of review
- 27. Process Validation- Oral suspensions Focuses on API micronization processes (if applicable) colloidal milling process (as applicable), homogenization filling Viscosity, fill volume/weight variation, Other critical attribute that may be affected by filling process? Other parameters as per FPP spec including, PSD, pH, dissolution, Protocol with commitment is acceptable at the time of review
- 28. Process validation- sterile products Products mfd by Terminal sterilization Products mfd by Aseptic processing Container and component sterilization and depyrogenation - Depyrogenation by tunnel depyrogenator (e.g. ampoules) or washing (e.g. rubber stoppers, plastic bottles) - Depyrogenation by washing- for stoppers, seals, accessories* - Validation of steam sterilization – for stoppers, seals, accessories* - Dry heat sterilization and depyrogenation- for glass vials or ampoules*
- 29. Process validation- sterile products- Contd Products mfd by Terminal sterilization Products mfd by Aseptic processing Product sterilization Terminal sterilization by Steam sterilization, radiation or ETO (as applicable)* Filter validation (as part of dev’t pharm) Process simulation - Media fill Full batch processing (other aspects of the mfg process, e.g. valdn of bulk prepn, filling and sealing quality) 3 production batches mfd at proposed scale 3 production batches mfd at proposed scale (commitment may also be accepted). *validation should be on three runs to demonstrate reproducibility.
- 30. clinical/BE batch- solids and suspensions (as part of process validation) A good check point to verify performance relative to the biobatch All validation batches should be profiled in the routine media on 12 units, using time points as used for biobatch Comparison with historical biobatch profile, with calculation of f2 (as necessary), should be performed and results discussed Check if the protocol includes adequate instruction/provision
- 31. Matrixing/bracketing approach Multiple strengths of same product (common blend) until stages of final granules: 3 consecutive batches of the common blend (instead of 3 separate blend batches for each strength) compression: 3 consecutive batches of each strength Primary packaging of tablet/capsule products blistering of hygroscopic or moisture sensitive products however should always be individually validated
- 32. Process validation- commitment As described in Annex 4,TRS 970, applicants are not expected to have process validation data before PQ In this case satisfactory PV protocol (PVP) and appropriately worded commitment are essential PVP or signed commitment letter should clearly indicate the need for prospective validation as finalized on three consecutive production batches, unless other wise justified.
- 33. Retrospective validation for established products Generally acceptable if condition described in Annex 4,TRS 970 (generic guide), are met. Tries to demonstrate process effectiveness and consistency via trend analysis: extent of deviations extent of OOS or OOT extent of batch rejection extent of product complains extent of changes/ improvements introduced SeeAppendix 2 of Annex 4,TRS 970
- 34. Review of protocol- main aspects to check Scope of the validation (type, batch size, reason)- do they reflect the planned validation? Highest batch size to be validated? Major equipments identified (in line with BMR) and a provision for recording their Q status included? Reference to current master production record included? Summary of critical steps identified? is this convincing ? Monitoring and sampling plan provided?- Do you agree with the steps monitored/sampled? Sampling schedule, schematics, tests and acceptance criteria, as well as current specification codes included ? Are these acceptable?
- 35. Review of protocol- main aspects to check-contd For solid orals: final blending, compression/encapsulation, coating stages must be adequately sampled and tested.Are these being reflected? Blend uniformity: Sampling schemes and blend uniformity acceptance criteria specified?Are these acceptable? Compression/encapsulation at lower, target and upper speeds included? Provision for performance of dissolution profile testing and comparison with the biobatch included? Appropriate commitment (prospective validation on first three consecutive batches mentioned) provided? Protocol reference and version number included in QIS?
- 36. Review of validation report Is the reported data relevant for the proposed manufacturing process and scale equipment used, process parameters applied All critical steps adequately monitored/sampled? Level of sampling and size are acceptable? All results within acceptable limits? Particular trend? Deviations appropriately evaluated and discussed? Is the overall process in sufficient control? Is there any thing that should be improved or refined for future production batches
Editor's Notes
- #15: Normally, the protocol should require validation of the BMR range, but once validation is executed either the BMR set up parameters should be revised to reflect the validated range or set point or new validation should be done.
- #26: i.e, in sigma process, the whole area within +/-3 of SD will be within spec limit, while in case of 4 sigma, +/4 SD of the curve area is within spec limit (and in this case Cpk is roughly equal to 1.33).