Protein/DNA Sample preparation methods
- 2. Introduction • Sample is a small part or quantity intended to show what the whole is like. • Following as sample can be used for laboratory diagnosis of leishmaniasis. i. Cells/Tissues ii. DNA from tissues iii. Blood or Urine. • Tissues can either be examined under microscope for presence of parasite or DNA from tissues can be examined, while blood can be used to test the presence of immunoglobulins, proteins or other components. Several steps involved in sample preparation are based on type of sample and mode of test. (S.Sundar,Rai.M; Clin Diagn Lab Immunol, 2002. PubMed Central)
- 3. Protein sample preparation Extraction of the entire protein from serum requires a multi –step protocol, where various sample processing steps are performed to increase efficacy of the extraction procedure. In case of bacterial/ animal/plant protein extraction- sample collection, stabilization, and protein extraction. After separation of serum from whole blood or lysis of cell (bacterial/animal/plant) cell lysate, other components must be removed by filtration, or centrifugation. Cells or tissue are disrupted in such a way as to minimize proteolysis and other modes of protein degradation. Lysis of these cells could be done using- Osmotic shock, Enzyme digestion, Detergent ,Grinding or UltraSonicator. It should be performed at as low temperature as possible and with a minimum of heat generation and even using protease inhibitors will give good results.
- 4. Separation of plasma 1) Blood is collected into purple top EDTA tubes and centrifuged (2000 rpm) at 4 degrees centigrade for 20 minutes. 2) 2) After centrifugation place 1.0ml of plasma into 1.5ml eppendorf tube and label it. 3) 3) Freeze immediately at –80 degree freezer. Separation of Serum 1. A 10 ml tube of whole blood is collected following standard procedures using a serum separator tube (Golden, tiger top tube) from each patient. 2. 2. Allow samples to clot for one hour at room temperature 3. 3. Centrifuge for 10 minutes at approximately 4000 rpm 4. 4. Aliquot serum into labelled cryovials. 5. 5. Immediately freeze vials of serum at –80-degree freezer For sample proteins from Blood Source: Rutgers Cancer institute of New Jerzy-protocol
- 5. Preparation of lysate from cell culture 1. Placed the cell culture dish on ice and wash the cells with ice-cold PBS. 2. Then ice-cold lysis buffer is added (1 mL per 107 cells/100 mm dish/150 cm2 flask; 0.5 mL per 5x106 cells/60 mm dish/75 cm2 flask). 3. Gently transfer the cell suspension into a pre-cooled microcentrifuge tube. 4. Maintain constant mixing for 30 min at 4°C. 5. Centrifuge in a microcentrifuge at 4°C for 20 min at 12,000 rpm. 6. Gently remove the tubes from the centrifuge and place on ice, aspirate the supernatant and place in a fresh tube kept on ice. Discard the pellet. For sample proteins from Cells/Tissues Source: Abcom protocol : protein sample preparation
- 6. Extraction methods are divided in three major types- Gentle, Moderate, and Vigorous. For sample proteins from Cells/Tissues Contd.. Gentle • Cell Lysis (osmotic Shock) • Enzyme digestion • Detergent digestion • Hand homogenization • Mortar pestle grinding Moderate • Grinding with glass beads • Blade homogenization • Freeze/Thaw Vigorous • Ultrasonication • French Press Source: G E Healthcare life sciences protein extraction handbook
- 7. Protein Purification Proteins can be purified using techniques based on molecular size , solubility of proteins, or charge. Based on Molecular size- Density gradient centrifugation, Size-exclusion chromatography. Based on charge- Ion-exchange chromatography electrophoresis Based on Solubility of Proteins- Using Salt (NaCl, MgCl2 ) at different concentration
- 8. Density gradient centrifugation Protein containing solution is suspended in uniform density gradient of sucrose and sedimentation of protein occurs in tube depending upon its rate of sedimentation. Sedimentation depends upon weight, density and size on the protein. Some other centrifugation technique like differential sedimentation centrifugation, Source: Sigma Aldrich
- 9. Chromatography Chromatography is a technique for separating mixture into their components in order to analyze, identify, purify the components. It is based on the separation of components based on their relative affinity or solubility to different phages of chromatography. i.e. Mobile phase (M.P.) and Stationary phase(S.P.). The different types of combination of stationary and mobile phases are used for different types of chromatography, which is selected based on our requirement. Types of chromatography I. Paper chromatography – for dried liquid samples with a liquid solvent and paper as S.P. II. Thin Layer chromatography – for dried liquid samples with liquid solvent and thin layer of stationary phase (alumina or silica gel on glass plate) III. Liquid chromatography - for Liquid samples with liquid solvent and solid beads. IV. Gas chromatography - mainly used for vaporized or volatile samples with carrier gas and S.P. of Liquid or solid beads. V. HPLC – mainly for already purified protein, used for analytical purposes.
- 10. Gel-filtration chromatography Porous particles are used to separate molecules of different size. Proteins passed over a column filled with a hydrated porous beads made of a carbohydrate or polyacrylamide polymer. Mixture of protein dissolved in suitable buffer, is allowed to flow by gravity down the column. Very large molecules cannot penetrate into the pores of the beads without entering the pores, the small molecules enter the pores. Large molecules are excluded and small proteins are retarded due to pores. This way separation on the basis of size and weight is done. Source: www.mikeblaber.org/lecture 31
- 11. Ion-exchange chromatography Separation of proteins in a column filled with charged polmer beads. Positively charged beads- anion exchange chromatography Negatively charged beads- cation exchange chromatography Proteins with opposite charge binds to the beads respectively. More charged protein will bound well and so less charged proteins will be eluted first. Finally bound proteins can be eluted using salt. Source: mastering-biochemistry.com
- 12. Gel Electrophoresis Macromolecules ( proteins, nucleic acids) are separated under the electric field on the basis of their size and charge. Voltage of approx. 70- 100 volts is used, which may vary depending upon the sample and gel size. Different types of electrophoresis are performed on the basis of molecules. Molecules move towards opposite charged electrode and hence get resolved. The movement of molecule also depend on size, shape, and applied voltage. Polyacrylamide or agarose gels are used for caring sample and for resolution of their components. SDS (sodium dodecyl sulfate) is anionic detergent which bind to peptide chain and provides uniform negative charge, and so cause proteins move to cathode.
- 13. PAGE Poly Acrylamide gel electrophoresis is mainly performed for protein samples. It is mainly of two types- Native PAGE and, SDS-PAGE. In Native PAGE the protein is separated in their native form or conformation, however in SDS-PAGE. As SDS is an anionic detergent which breaks polymeric proteins to their monomeric subunits and linearize them. Image Source: www.bio-rad.com- literature bulletin
- 14. Image Source: www.bio-rad.com- literature bulletin
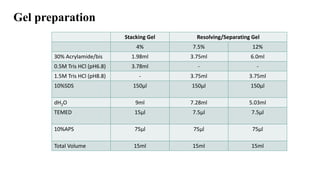
- 15. Gel preparation Stacking Gel Resolving/Separating Gel 4% 7.5% 12% 30% Acrylamide/bis 1.98ml 3.75ml 6.0ml 0.5M Tris HCl (pH6.8) 3.78ml - - 1.5M Tris HCl (pH8.8) - 3.75ml 3.75ml 10%SDS 150µl 150µl 150µl dH2O 9ml 7.28ml 5.03ml TEMED 15µl 7.5µl 7.5µl 10%APS 75µl 75µl 75µl Total Volume 15ml 15ml 15ml
- 16. Buffer preparation Components/L volume Tris Base 30.4 g Glycine 144.2 g SDS 10.0g The volume was made up to 1L with water Running Buffer (10X) Components/L Concentration Tris HCL pH 8 50 mM NaCl 150 mM NP-40 1% Sodium deoxycholate 0.5 % SDS 0.1 % Lysis Buffer Laemmli Buffer Tris-Cl 60mM Glycerol 10% SDS 3% Bromophenol blue 0.05% ᵝ- mercaptoethanol 10% TAE Buffer Ccomponents Main Composition 50X 1X Tris Base 2 M 242.2 g/l 4.844 g/l Acetic Acid 1 M 60.5 ml/l 1.21 ml/l EDTA Sodium salt dihydrate 50 mM 18.612 g/l 0.372 g/l
- 17. DNA Isolation DNA is a very long molecule made up of a chain of nucleotides and the order of these nucleotides is what makes organisms similar to others of their species and yet different as individuals. Genes are sections within this long DNA molecule. In eukaryotic cells, DNA is organized as chromosomes in an organelle called the nucleus. Bacterial cells have no nucleus, so their DNA is organized in rings or circular plasmids, which are in the cytoplasm. The DNA extraction process frees DNA from the cell and then separates it from cellular fluid and proteins so you are left with pure DNA. The three basic steps of DNA extraction are 1) lysis, 2) precipitation, and 3) purification.
- 19. Extraction Protocol 1. Take 3ml of whole blood in Falcon tube. 2. Add 2ml of reagent A. 3. Mix well at room temperature. 4. Centrifuge at 10000rpm for 5 minutes at room temperature. 5. Discard supernatant carefully and add 1ml of reagent B. 6. Vortex briefly for better mixing. 7. Place tube in water bath for 15 min at 65 degree Celsius. 8. Allow to cool at room temperature and add Phenol : Chloroform : isoamyl alcohol. 9. After proper mixing, centrifuge at 12000pm for 2 min. 10. Transfer upper phase to new tube and add 2ml of ice cold ethanol and invert mix well. 11. Spool the cottony DNA using pipette. 12. Air dry and resuspend in TAE buffer for further use.
- 20. Reagents Reagent A- for RBC lysis 0.01M Tris-HCl pH 7.4 320mM Sucrose 5mM MgCl2 1% Triton X100 Preparation- Add 10 ml of Tris,109.54g of sucrose, 0.47g of MgCl2,and 10 ml of Triton X-100 to 800ml of distilled water and adjust pH to 8.0. make final volume to 1 L. Reagent B- for cell lysis 0.4M tris-HCl, 150 mM NaCl, 0.06M EDTA, 1% sodium dodecyl sulfate , pH 8.0 preparation- Take 400 mL of Tris, 120 mL of EDTA, 8.76g of NaCl, and adjust pH, final volume to 1L.
- 21. The obtained DNA can be then used for PCR, or Electrophoresis. For electrophoresis the DNA sample is mixed with Loading dye and added to well for allowing it to resolve on gel. For PCR-(for amplification of DNA ) A mix of templet DNA , dNTPs, Polymerase Enzyme and Primes, and allow it to step by step Denaturation, Annealing, and Elongation. The PCR product could be checked for amplification on Agarose gel electrophoresis.
- 22. Thank You !