Quality control and inspection
- 1. PRESENTED BY: SAMIKSHA B.SAWANT M,PHARM (IP), 1ST SEM GUIDED BY: DR. INDIRA PARAB
- 2. “QUALITY IS NOT AN ACCIDENT; BUT IT IS THE RESULT OF INTELLIGENT EFFORTS”
- 3. WHAT IS QUALITY? “Quality is fitness for use. ” “The totality of features and characteristics of a product or service that bear on its ability to satisfy a given need.” “Quality involves meeting customers need, preferences and exceeding it.” “Quality also encompasses people, process and environment.”
- 4. WHY QUALITY CONTROL? Manufacturing process is a repetitive process depending on both controllable and non- controllable factors. This produces deviation in the quality of the product. QC is the process of verification , or correction of the quality of the product when deviations are found to be more than expected.
- 5. WHAT IS QUALITY CONTROL? “Those planned and systematic actions which provides a mean to control and measure the characteristics of a product, process or a service to established requirements.”
- 6. QUALITY CONTROL AS PER ISO: “The operational techniques and activities that are used to satisfy quality requirements.” The quality control system verifies and maintains desired level of quality in an existing product or service by careful planning, use of proper equipments and continued inspection and corrective action as required.
- 7. WHAT IS QC INSPECTION The ISO standard defines inspection as “activity of measuring, examining, testing one or more characteristics of a product or service and comparing the results with specified requirements in order to establish whether conformity is achieved for each characteristic.”
- 8. QUALITY INSPECTION LOOP IDENTIFICATION OF DEFECTS FEEDBACK OF THIS DEFECT TO APPROPRIATE PERSON DETERMINATION OF CAUSE OF DEFECT CORRECTION OF DEFECT QC AND INSPECTION
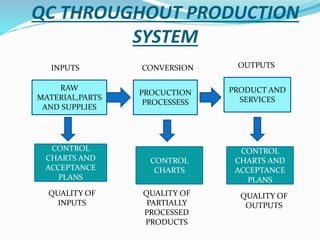
- 9. QC THROUGHOUT PRODUCTION SYSTEM RAW MATERIAL,PARTS AND SUPPLIES PROCUCTION PROCESSESS PRODUCT AND SERVICES CONTROL CHARTS AND ACCEPTANCE PLANS CONTROL CHARTS CONTROL CHARTS AND ACCEPTANCE PLANS INPUTS CONVERSION OUTPUTS QUALITY OF INPUTS QUALITY OF PARTIALLY PROCESSED PRODUCTS QUALITY OF OUTPUTS
- 10. The safety and efficacy of the finished dosage form is largely dependent on the purity and quality of the bulk active drug substance. Physical tests such as particle size for raw materials flow properties etc. are essential tests to assure consistent operation of the production and control system and to assure quality and efficacy PRE-PRODUCTION QC INSPECTION
- 11. PRE-PRODUCTION QC INSPECTION To decrease quality risk, the inputs can be inspected prior to production. Samples are randomly taken and checked. An experienced inspector examines the sample/prototype to make sure that the raw materials meet the specified standards Whether development team has clearly communicated the requirements to the manufacturing team. Whether equipments for mass production is similar to that for making prototypes.
- 12. IN PROCESS INSPECTION The first products that got out of the line are inspected for conformity. If issues are raised at this stage, the factory can immediately take actions and avoid delays. In-process products are rarely checked as it takes technician to reliably detect errors on unfinished products.
- 13. IN PROCESS QC INSPECTION Inspect the test results from in-process tests performed for conformance with established sampling and testing protocols, analytical methods, and specifications. For example, evaluate the tests for weight variation, hardness, and friability. All testing must comply with cGMP. The inspection must confirm that the in-process tests were done, as described in the plan, and ascertain that the results are within specifications.
- 14. CLASSIFICATION OF IN-PROCESS INSPECTION 1. Trial run inspection: Tools and machines are checked before operation. 2. First-off inspection: The items produced in the first production run are inspected and examined with respect to specifications. 3. Inspection by self control: Done by operators, controlling operations at different levels of production process. 4. Decentralised inspection: Semi finished goods are inspected either on machines or in the production line.
- 15. 5. Centralised inspection: There can be single inspection unit for whole plant Or each section can have inspection unit to inspect the items The inspection staff is more experienced and skilled in this case Sophisticated and reliable instruments and techniques are use to measure the quality Hence centralised inspection is reliable and accurate.
- 16. QC INSPECTION IN PRODUCTION 1) Component dominant: Incoming material must be checked for required specifications. 2) Set-up dominant: An operation once set at a level, remains at that level for long. Hence products produced initially if found free from defects and conforming to specifications, then the operation can be cleared for continuous operation. 3) Machine dominant: Operation drift away from initial set-up level as operation proceeds. Hence needs periodic inspection for correcting set up.
- 17. 4. Operator dominant: A certain portion of job is entirely influenced by operator’s skill. 5.Information dominant: All the information including the SOP’s, nature of job is given to concerned person. 6.Record dominant: The written records and documentation of every process and test conducted should be maintained.
- 18. QC INSPECTION IN ANALYTICAL In general, these inspections include: The specific methodology which will be used to test a new drug product A complete assessment of laboratories conformance with GMP’S A specific aspect of laboratory operations
- 19. Laboratory records and logs represent a vital source of information that allows a complete overview of the technical ability of the staff and of overall quality control procedures. SOPs should be complete and adequate and the operations of the laboratories should conform to the written procedures. Specifications and analytical procedures should be suitable and, as applicable, in conformance with application commitments and compendial requirements
- 20. Documents relating to the formulation of the product, synthesis of the bulk drug substance, product specifications, analysis of the product, and others are examined during the review process in headquarter Inspections are designed to determine if the data submitted in an application are authentic and accurate and if the procedures listed in the application were actually used to produce the data contained in the application. Additionally, they are designed to confirm that plants (including QC laboratory) are in compliance with CGMP regulations.
- 21. Based on team inspection approach. Highly technical and specialised testing equipments, procedures, data manipulations as well scientific laboratory operations will be evaluated. The inspection of a laboratory requires the use of observations of the laboratory in operation and of the raw laboratory data to evaluate compliance with CGMP's. FDA INSPECTION
- 22. FDA INSPECTION- 4M’s 1. MACHINE: Inspection should confirm that preventive maintenance, cleaning, adjustment etc are performed Machine usage, maintenance, calibration logs, repair records should be examined Verify that the equipments were in good working order at the time the batches were analysed.
- 23. 2. METHOD/PROCESS: Information regarding validation of methods should be carefully evaluated All processes that may cause deviation to a device’s specification and all validated process must be monitored and controlled If the process is software controlled, confirm that the software was validated Review the software documents, software validation activities, software change controls and software validation results to confirm that software will meet user need
- 24. 3. MATERIALS: Raw material testing is of utmost importance as it directly affects the quality of final product Hence inspection should examine the analysis of materials including purity test, quality, charts etc Inspect if the methods for analysing the purity were validated The manufacturer must have complete knowledge of manufacturing process and the potential impurities that may appear in materials. These impurities cannot be evaluated by without a suitable method and one that has been validated
- 25. 4. MAN: Verify that personnel have been qualified to implement validated processes or appropriately trained to implement processes which yield results that can be fully verified Confirm that the employees have complete knowledge of the devices, processes Confirm that employees are aware of the device defects that may occur as a result of improper performances Confirm that the employees conducting QC tests are aware of the defects and errors that may be encountered while performing their responsibilities
- 26. FINAL INSPECTION It is also known pre-shipment inspection. This is the most popular type of QC inspection for importers. It takes place once all the products are finished and ready for shipment. The samples are drawn in a random manner and thus can be representative of the whole batch.
- 27. STATISTICAL QUALITY CONTROL It is a technique for controlling quality of product using a set of statistical tools It involves two elements: Statistical process control: This summarizes collection of data , makes use of control charts. Acceptance sampling
- 28. CONTROL CHARTS Primary purpose of control charts is to indicate when production processes might have changed sufficiently to affect product quality. If the indication is that product quality has deteriorated, corrective is taken. Compare attributes (No. of defectives in a sample)or variables (characteristics that can be measured on a continuous scale (weight, length, etc.) of the sample with that of the standard
- 29. ACCEPTANCE SAMPLING OR SAMPLING INSPECTION It is the process of evaluating portion of the product material in a lot for the purpose of accepting or rejecting the lot as either conforming or not conforming to quality specifications. The acceptance plan identifies the: Size of samples, n Type of samples Decision criterion, c, used to either accept or reject the lot Samples may be either single, double, or sequential.
- 30. SINGLE SAMPLING PLAN Draw random sample (n) from the lot Determine number of defection in the sample If total no. of defects does not exceed c, accept the lot If defection exceed c, reject the lot
- 31. DOUBLE SAMPLING PLAN Determine no. of defectives in the sample(n) Defectives do not exceed c1, accept the lot Defectives equal or exceed r1, reject the lot Defectives exceed c1 but less than r1, draw another sample(n2) Determine no. of defectives in second sample Total no. of defectives equals or exceeds c2, reject the lot Total no. of defectives does not exceed c2. accept the lot
- 32. SEQUENTIAL/MULTIPLE SAMPLING This is extension of double sampling plan At each stage of sampling the cumulated results are analysed to take decision of accepting/rejecting the lot If no final decision can be taken at anyy stage, then another sample is drawn to take further decision.
- 33. QUALITY AUDIT ISO defines audit as systematic and independent examination to determine whether quality activities and related results comply with planned arrangements and whether these are implemented effectively and are suitable to achieve objectives. It checks if quality system and procedures are Free from congenital defects Are capable of achieving and maintaining standards of quality chosen by enterprise or costumers being adhered to and compiled with, in day to day work.
- 34. QUALITY AUDIT AND FOLLOW UP Prior to writing auditing report, auditor explains the observations to auditee Corrective actions to be taken are proposed Audit report are written in standard format which contain area audited, dates of audit, persons contacted, commendable features and recommendations. The report must contain status of implementation of pending corrective measures as per previous audit.
- 35. QC INSPECTION IN DISTRIBUTION AND STORAGE GMP summarises following principles with respect to distribution: Only authorised products are distributed Premises are suitable for their intended use and kept on good sanitary condition All products are received, stored and handled carefully All operations are performed according to written procedure, supervised and documented Adequate provision exist to handle complaints, recalls and return goods
- 36. Storage: Warehouse should be clean, inaccessible for unauthorised persons, temperature and humidity control, adequate shelving, free from insects and vermin. Special storage: Availability of cold room/refrigerator for vaccines and biological products Special storage areas for controlled drugs and other prescription drugs Suitable and secure storage facility for controlled drugs and poisons
- 37. INSPECTION OF ESTABLISHMENT INSPECTION OF THE DRUG DISTRIBUTION CHAIN Objectives: To ensure- Protection of patients and members of the public from malpractice by distributors and supplier of drugs Adherence to the drug laws and regulations governing compounding, distribution, importation, export and storage of drugs. High ethical and professional standards of pharmaceutical practice.
- 38. INSPECTION AND NARCOTIC SECTION Functions of Narcotic Drug Unit: Controls the use of narcotic and pyschotropic drug substances Issue importation and trade license for narcotic drugs Prepare registry book and template necessary to control use of narcotic substances and distribute them among the concerned authority Prepare annual statistic on narcotic and pyschotropic drug substances.
- 39. Production area of narcotic drugs should be isolated from other production area The areas should be such that they preclude the drugs falling into hands of unauthorized persons The rooms should be equipped with security alarms Room should have metal door and be lockable and non passsable The room shall be without windows and separated from surrounding rooms by partitions Separate record of all activities related to these drugs must be maintained.
- 40. PENICILLIN DRUGS Dedicated, self contained premises, facilities and equipments must be used Dust generating operations should maintain relatively negative pressure The exhaust air should be decontaminated as required Production area should be ventilated and temperature, humidity and filtration should be controlled Separate storage area should be maintained and the area must be clearly marked Its access is restricted to authorised persons QC labs should be designed to suit intended use and avoid mix-ups There should be adequate space for sample handling, storage of retention and stability samples and storage of records.
- 41. HORMONAL PRODUCTS The premise should be designed to facilitate cleaning and decontamination Various rooms should have interlock mechanism or other system to prevent opening of more than one door at a time Supply of safe breathing air should be provided to prevent the operate from inhaling air from the facility This can be done by central air supply system or self contained breathing apparatus Alarm system for: main air supply failure, temperature out of specification(oos), humidity oos, CO2 oos, CO oos and SO2 oos.
- 42. CONCLUSION Quality is a never ending prospect..... Increase in quality, increases production, decreases cost and increases profits Thus quality control and inspection form an integral part of a company’s management.
- 43. REFERENCES Quality management by Bholanath Das, published by New Century, pg 1, 80-82. Total quality management by G.R.Basotia, Published by Mangal Deep Prakashan, pg 69-73,118-119,125-128 Managing quality concepts and task by V. Narayana, and N.S. Sreenivasan, Published by H. S. Poplai for New Age International Limited, pg 66-69, 73-75 Fundamentals of quality control and improvement by Amitava Mitra, second edition, Published by PHI Learning Private Limited, pg 235-249, 421-479
- 44. REFERENCES TeamBuilding and Total Quality by Gene H. Milas, published by Narosa publishing house, pg 32, 74, 229 Total Quality Management by Dr J M Juran, volume 1, published in India by D.L. Shah Trust. Total Quality and Human Resource by Cooper, first edition, Published by Maya Publishers Pvt.Ltd, pg 235 Total Quality Management by S. Rajaram and M. Sivakumar, pg 83-194
- 45. REFERENCES Quality Planning and Analysis by J, M, Juran and Frank M. Gryna, second edition, Published by Tata Mc Graw- Hill publishing company limited, pg 356 http://apps.who.int/prequal/info_general/documen ts/TRS885/WHO_TRS_885-Annex6.pdf http://www.fda.gov/ICECI/Inspections/InspectionG uides/ucm074918.htm) http://qualityinspection.org/qc-inspection/) http://www.riigiteataja.ee/../narko_ja_ps M.indiamart,com/.../anti-cancer-medicine..
- 46. THANKYOU