REACTIONS.of organic chemistry pptx (.).
- 2. GROUP MEMBERS BUDER UN NISA MAHNOOR ALI FIZA AKBAR
- 3. CONTENT Baeyer-Villiger Oxidation Diels Alder Reaction Wolff Kishner Reduction Friedel Craft’s Reaction Perkin Reaction Cannizzaro’s Reaction
- 4. Baeyer-Villiger Oxidation The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899. The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant.
- 5. EXAMPLES
- 6. MECHANISM The peroxyacid protonates the oxygen of the carbonyl group. The peroxyacid attacks the carbon of the carbonyl group forming what is known as the Criegee intermediate One of the substituents on the ketone group migrates to the oxygen of the peroxide group while a carboxylic acid leaves. This migration step is thought to be the rate determining step.] Finally, deprotonation of the oxocarbenium ion produces the ester.
- 8. Diels-Alder Reaction The Diels-Alder reaction is an organic reaction that is used to convert a conjugated diene (a molecule with two alternating double bonds) and a dienophile (an alkene) to a cyclohexene. This process is concerted, where bonds form and break at the same time, and the entire reaction takes place in one step in the presence of heat. The class of reactions to which Diels-Alder belong is termed as cycloaddition.
- 9. EXAMPLES
- 11. Applications The Diels-Alder reaction is used in the synthesis of natural products like rubber and plastic. It also finds its application in pharmaceuticals and biomedical engineering. It is used to make synthetic steroids, such as cortisone and Vitamin D.
- 12. Wolff Kishner Reduction Aldehydes and ketones can be converted to a hydrazine derivative by reaction with hydrazine. These "hydrazones" can be further converted to the corresponding alkane by reaction with base and heat. These two steps can be combined into one reaction called the Wolff-Kishner reduction which represents a general method for converting aldehydes and ketones into alkanes.
- 13. 1. Reaction of Aldehydes or Ketones with Hydrazine Produces a Hydrazone 2. Reaction with a Base and Heat Converts a Hydrazone to an Alkane 3. Both Reactions Together Produces the Wolff-Kishner Reduction
- 14. MECHANISM 1. Deprotonation of nitrogen
- 15. 2. Protonation of the carbon 3. Deprotonation of nitrogen
- 16. 4. Protonation of carbon EXAMPLE:
- 17. Friedel Craft’s Reaction A Friedel-Crafts reaction is an organic coupling reaction involving an electrophilic aromatic substitution that is used for the attachment of substituents to aromatic rings. The two primary types of Friedel-Crafts reactions are the alkylation and acylation reactions.
- 18. Friedel Craft’s alkylation reaction Friedel-Crafts Alkylation refers to the replacement of an aromatic proton with an alkyl group. This is done through an electrophilic attack on the aromatic ring with the help of a carbocation. The Friedel-Crafts alkylation reaction is a method of generating alkylbenzenes by using alkyl halides as reactants.
- 19. MECHANISM OF ALKYLATION Step 1 The Lewis acid catalyst (AlCl3) undergoes reaction with the alkyl halide, resulting in the formation of an electrophilic carbocation. Step 2 The carbocation proceeds to attack the aromatic ring, forming a cyclohexadienyl cation as an intermediate. The aromaticity of the arene is temporarily lost due to the breakage of the carbon-carbon double bond. Step 3 The deprotonation of the intermediate leads to the reformation of the carbon-carbon double bond, restoring aromaticity to the compound. This proton goes on to form hydrochloric acid, regenerating the AlCl3 catalyst.
- 21. Friedel Craft’s acylation reaction The Friedel-Crafts acylation reaction involves the addition of an acyl group to an aromatic ring. Typically, this is done by employing an acid chloride (R-(C=O)-Cl) and a Lewis acid catalyst such as AlCl3. In a Friedel-Crafts acylation reaction, the aromatic ring is transformed into a ketone.
- 22. Perkin reaction The Perkin reaction can be considered as a type of condensation reaction. The Perkin reaction gives an alpha, beta-unsaturated aromatic acid via the aldol condensation of an aromatic aldehyde and an acid anhydride. The alkali salt of the acid is also present. This alkali salt acts as a base catalyst. Other bases can be used instead of the alkali salt of the acid in the Perkin reaction.
- 23. EXAMPLE:
- 24. APPLICATIONS One of the most important applications of the Perkin reaction is the laboratory synthesis of phytoestrogenic stilbene resveratrol. It is used in the laboratory to synthesize cinnamic acid. Cinnamon and shea butter both include cinnamic acids, which are naturally occurring unsaturated aromatic carboxylic acids. We may employ this reaction to create – and -unsaturated aromatic acids, which are commonly used in the pharmaceutical industry.
- 25. Cannizzaro’s Reaction Cannizzaro's reactions involves the base-induced disproportionation of two molecules of a non-enolizable aldehyde to yield a carboxylic acid and a primary alcohol Cannizzaro Reaction Mechanism details the method to get one molecule of alcohol and one molecule of carboxylic acid from two molecules of a given aldehyde.
- 26. EXAMPLE:
- 27. MECHANISM A nucleophile attack the carbonyl group of given aldehyde and giving rise to an anion carrying 2 negative charges.
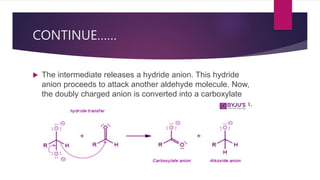
- 28. CONTINUE…… The intermediate releases a hydride anion. This hydride anion proceeds to attack another aldehyde molecule. Now, the doubly charged anion is converted into a carboxylate anion and the aldehyde is converted into an alkoxide anion.
- 29. CONTINUE…… In this final step, water offers a proton to the alkoxide anion which gives rise to the final alcohol product. The reaction can proceed since the alkoxide is more basic than water. Now, the carboxylate ion gives rise to the final carboxylic acid product.





![MECHANISM
The peroxyacid protonates the oxygen of the carbonyl group.
The peroxyacid attacks the carbon of the carbonyl group
forming what is known as the Criegee intermediate
One of the substituents on the ketone group migrates to the
oxygen of the peroxide group while a carboxylic acid leaves.
This migration step is thought to be the rate determining
step.]
Finally, deprotonation of the oxocarbenium ion produces
the ester.](https://image.slidesharecdn.com/reactions-231214172104-1617c46c/85/REACTIONS-of-organic-chemistry-pptx-6-320.jpg)