Types of corrosions
- 2. TYPE 1
- 3. 1. Uniform Corrosion This corrosion is also called General Corrosion. Effect produced by most direct chemical attacks. This is common form of corrosion. This type of corrosion is first seen as a general dulling of the surface and, if allowed to continue; the surface becomes rough.
- 4. How to prevent uniform corrosion? Uniform corrosion or general corrosion can be prevented through a number of methods: Use thicker materials for corrosion allowance Use paints or metallic coatings such as plating, galvanizing or anodizing Use Corrosion inhibitors or modifying the environment
- 5. Images
- 6. TYPE 2
- 7. 2. Galvanic Corrosion Also called as “dissimilar metal corrosion” Takes place when two metals are in physical contact with each other and are immersed in a conducting fluid. Corrosion damage induced when two dissimilar materials are coupled in a corrosive electrolyte.
- 8. Examples Plate and screw of different electrical potentials due to differences in processing Multiple component implant using different metals for each component Copper and steel tubing are Joined in a domestic water heater, the steel will corrode in the vicinity of the junction
- 9. The relative nobility of a material can be predicted by measuring its corrosion potential. The well known galvanic series lists the relative nobility of certain materials in sea water. A small anode/cathode area ratio is highly undesirable. In this case, the galvanic current is concentrated onto a small anodic area. Rapid thickness loss of the dissolving anode tends to occur under these conditions. Galvanic corrosion problems should be solved by designing to avoid these problems in the first place. Galvanic corrosion between stainless steel screw and Aluminium. Galvanic corrosion between Steel and Brass.
- 10. Note that the area ratio of the anode: cathode is an important variable affecting the dissolution current density (and hence corrosion rate) pertaining to the anode. The area ratio is also important when considering the relative amount of current "available" from the cathodic reaction. The following have been described as "main factors" influencing galvanic corrosion rates in Skanaluminium's on- line publication "Alubook - Lexical knowledge about aluminium". • Potential Difference between materials • Cathode Efficiency • Surface areas of connected materials (area ratio) • Electrical resistance of the connection between the materials and of the electrolyte. Fig. Brass on Weathering Steel - rust forms in discrete crystallites that are fine, red and diffusely reflecting, like hematite. The massive re-crystallized layer is a shiny blue, approaching the blue-black of secular hematite.
- 11. TYPE 3
- 12. 3. Differential Aeration Corrosion This type of corrosion takes place when a metal is unevenly exposed to different oxygen/air concentrations. The part which is exposed to less oxygen undergoes corrosion.
- 13. Differential Aeration Corrosion (Cont) At the anode (less O2 concentration), M ------------Mn+ + ne At the cathode (more O2 concentration), H2O + ½ O2 + 2e------ 2OH-
- 14. Examples Drop Corrosion: It takes place when a drop of electrolyte is in contact with the metal surface. The metal surface covered by is in contact with lesser amount of air than the uncovered metal surface. Thus, metal covered by drop becomes anodic and corroded whereas the
- 15. Waterline Corrosion Waterline corrosion s a case of differential aeration corrosion, more prevalent in cases such as ocean going ships, water storage steel tanks, etc., in which a portion of the metal is always under water. The waterline corrosion takes place due to the formation of differential oxygen concentration cells. The part of the metal below the water line is exposed only to dissolved oxygen while the part above the water is exposed to higher oxygen concentration of the atmosphere. Thus, part of the metal below the water acts as anode and undergoes corrosion and part above the waterline is free from corrosion. A distinct brown line is formed just below the water line due to the deposition of rust.
- 16. TYPE 4
- 17. 4. Pitting Corrosion Pitting corrosion is a localized form of corrosive attack that produces holes or small pits in a metal. the bulk of the surface remains unattacked. Pitting is often found in situations where resistance against general corrosion is conferred by passive surface films.
- 18. 4. Pitting Corrosion (cont…) Localized pitting attack is found where these passive films have broken down. Pitting attack induced by microbial activity, such as sulfate reducing bacteria (SRB) also deserves special mention.
- 19. Pitting Corrosion (Images) Corrosion Pits are the primary source of leaks in water handling systems
- 20. TYPE 5
- 21. 5. Stress Corrosion This type of corrosion is observed in fabricated articles which are subjected to various mechanical operations Here mechanical operations refers Bending, Hammering and annealing. This corrosion is usually unpredictable is nature.
- 22. Examples Season Cracking Caustic embrittlement of mild steel
- 23. TYPE 6
- 24. 6. Corrosion Fatigue Corrosion Fatigue is a special case of stress corrosion caused by the combined effects of cyclic stress and corrosion. Control of corrosion fatigue can be accomplished by either lowering the cyclic stress or by corrosion control.
- 25. Images
- 26. TYPE 7
- 27. 7. Crevice Corrosion Crevice corrosion is a localized form of corrosion usually associated with a stagnant solution on the micro-environmental level. Such stagnant microenvironments tend to occur in crevices (shielded areas) such as those formed under gaskets, washers, insulation material, fastener heads, surface deposits, disbonded coatings, threads, lap joints and clamps.
- 28. Crevice Corrosion (cont..) Occurs under gaskets, rivets and bolts, between valve disks and seats. Well-known examples of such geometries including flanges, gaskets, disbonded linings/coatings, fasteners, lap joints and surface deposits.
- 30. TYPE 8
- 31. 8. Intergranular Corrosion Intergranular corrosion refers to preferential (localized) corrosion along grain boundaries. or immediately adjacent to grain boundaries, while the bulk of the grains remain largely unaffected. This form of corrosion is usually associated with chemical segregation effects (impurities have a tendency to be enriched at grain boundaries) or specific phases precipitated on the grain boundaries.
- 32. Intergranular Corrosion (cont…) This selective dissolution may lead to the dislodgement of grains. Intergranular corrosion in sensitized stainless steels and exfoliation in aluminum alloys represent industrially significant examples of this form of damage.
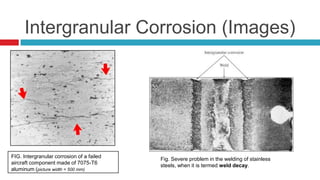
- 33. Intergranular Corrosion (Images) FIG. Intergranular corrosion of a failed aircraft component made of 7075-T6 aluminum (picture width = 500 mm) Fig. Severe problem in the welding of stainless steels, when it is termed weld decay.
- 34. Type 9
- 35. 9. Microbiological Corrosion Caused by microbes like bacteria, algae fungi etc These microbes can be aerobic or anaerobic Aerobic bacteria decrease the concentration of oxygen in the medium in contact with metal surface. The main product of corrosion in anaerobic corrosion is iron sulphide
- 37. Type 10
- 38. 10. Filiform Corrosion Occurs under painted or plated surfaces when moisture permeates the coating. Filiform corrosion is also known as "underfilm Corrosion" or "filamentary corrosion". Filiform corrosion can be visually recognized without using a microscopy
- 39. Prevention methods for Filiform Corrosion Filifrom Corrosion can be prevented with the following methods: control the relative humidity use brittle coatings
- 40. Images The mechanism of filiform corrosion is shown in the figure below.
- 41. TYPE 11
- 42. 11. Erosion Corrosion It is the result of relative movement between the corrosive fluid and metal surface All types of equipments exposed to moving fluids are subjected to erosion corrosion. Surface chemistry can play a role in erosion corrosion due to mechanochemical effects.
- 43. Erosion Corrosion Examples Ship propellors Hydralic turbines Pump impellers Diesel engine cylinder
- 45. TYPE 12
- 46. 12. Soil Corrosion The two factors which promote corrosion ie.., moisture and dissolved electrolytes are present in soil, making it corrosive. Presence of micro-organisms in soil further leads to corrosion of underground and pipeline.
- 48. TYPE 13
- 49. 13. Hydrogen Damage Hydrogen Damage is the process by which various metals, most importantly high-strength steel, become brittle and fracture following exposure to hydrogen. Hydrogen damage is often the result of unintentional introduction of hydrogen into susceptible metals during forming or finishing operations and increases cracking in the material. This phenomenon was first described in 1875.
- 50. Divisions in Hydrogen Damage Hydrogen damage can be divided into following types Hydrogen Blistering Hydrogen embrittlement Decarburization Hydrogen attack
- 52. TYPE 14
- 53. 14. Fretting Corrosion Fretting refers to wear and sometimes corrosion damage at the asperities of contact surfaces This corrosion occurs at the interface between contacting, highly loaded metal surfaces when subjected to slight vibratory motions is know an fretting corrosion.
- 54. Fretting Corrosion (cont..) This corrosion is most common in bearing surfaces in machinery. he most common type of fretting is caused by vibration. The protective film on the metal surfaces is removed by the rubbing action and exposes fresh, active metal to the corrosive action of the atmosphere.
- 56. TYPE 15
- 57. 15. Selective Leaching Selective leaching, also called dealloying, demetalification, parting and selective corrosion Selective leaching is a corrosion process in which one constituent of an alloy is preferentially dissolved by the environment, leaving the dealloyed metal weak and often porous
- 59. Overview