3.2
- 1. Thermal Physics Topic 3.2 Thermal Properties of Matter
- 2. Specific Heat Capacity The amount of thermal energy needed to raise the temperature of a mass of one kilogram of a substance by one kelvin is called the specific heat capacity, c. The thermal energy required to raise mass m with specific heat capacity c by a temperature ∆T is ∆Q =mc∆T
- 3. Heat Capacity/Thermal Capacity When substances undergo the same temperature change they can store or release different amounts of energy They have different Heat Capacities Heat capacity = ∆Q / ∆T in JK-1 ∀ ∆Q = the change in thermal energy in joules ∀ ∆T = the change in temperature in Kelvin Defined as the amount of energy to change the temperature of a body by unit temperature Applies to a specific BODY
- 4. Heat Capacity - 2 A body with a high heat capacity will take in thermal energy at a slower rate than a substance with a low heat capacity because it needs more time to absorb a greater quantity of thermal energy They also cool more slowly because they give out thermal energy at a slower rate
- 5. Specific Heat Capacity Defined as the amount of thermal energy required to produce unit temperature rise in unit mass of the MATERIAL Unit mass is normally 1kg, and unit temperature rise is normally 1K Specific Heat Capacity = ∆Q / (m∆T) in J kg -1 K-1 • where m is the mass of the material
- 6. For an object made of 1specific material then with specific heat capacity=c Heat Capacity = m x Specific Heat Capacity C=mc Q=C∆T
- 7. Example 1 When a car brakes, an amount of thermal energy equal to 112 500J is generated in the brake drums. IF the mass of the brake drums is 28 kg and their specific heat capacity is 460.5 J/kg*k, what is the change of temperature. ∆Q =mc∆T ∆T=∆Q /mc=112500/(28*460.5) =8.70
- 8. Specific Heat Capacity - 2 Unit masses of different substances contain • different numbers of molecules • of different types • of different masses If the same amount of internal energy is added to each unit mass • it is distributed amongst the molecules
- 9. Specific Heat capacity - 3 The average energy change of each molecule will be different for each substance Therefore the temperature changes will be different So the specific heat capacities will be different
- 10. Thermal Equilibrium When a cold body and a hot body are placed in contact, thermal energy flows from the hot body to the cold body until the two temperatures are the same. This is called thermal equilibrium. The energy lost by the hot object is equal to the energy gained by the cold object
- 11. Example 2 A piece of iron of mass 200g and temp. 3000 C is dropped into 1kg of water of temp. 200 C. What will be the temperature of the water become? ciron= 470J/kg*K, cwater=4200J/kg*k mici(300-t)=mwcw(t-20) T=260 C
- 12. Phases (States) of Matter Matter is defined as anything that has mass and occupies space There are 4 states of matter Solids, Liquids, Gases and Plasmas Most of the matter on the Earth in the form of the first 3 Most of the matter in the Universe is in the plasma state
- 13. Change of State Ordinary matter can exist as a solid, a liquid, or a gas Heating ice will turn it into water and heating water will turn it into steam In order for a piece of ice to melt it must be 0o C and in order to turn water into steam it must be 100o C
- 14. Change of State Once at the melting point, any additional thermal energy supplied does not increase the temperature. The thermal energy is used rather to overcome the forces between the water molecules in the ice The temperature of a substance will not change until it completely changes its state
- 15. Latent Heat The thermal energy which a particle absorbs in melting, vaporising or sublimation or gives out in freezing, condensing or sublimating is called Latent Heat because it does not produce a change in temperature
- 16. Specific Latent Heat Specific latent heat of fusion- Lf – is the thermal energy required to melt a unit of mass of material Specific latent heat of vaporization- Lv – the thermal energy required to vaporize a unit mass at its boiling point
- 18. Specific Latent Heat The term specific specifies that the thermal energy necessary to change the phase of a substance irrespective of mass
- 19. Specific Heat The energy required to produce a phase change is equal to the mass of the substance as well as the latent heat of fusion/vaporization. Q = mLf Q = mLv
- 20. Example 1 An ice cub of mas 25.0 g and temperature -10 C is dropped into a glass of water of mass 300.0 g and temperature 20 C. What is the temperature eventually? (Specific heat capacity of ice = 2200J/kg*k; Latent heat of fusion of ice = 334 kj/kg) 11.9 C
- 21. Example 2 Thermal energy is provided at a constant rate of 833J/s to 1kg of copper at the melting temperature. If it takes 4 minutes to completely melt the copper, find the latent heat of fusion of copper. Lf= 200 kJ/kg
- 22. Measuring Specific Heats The specific heat capacity of a solid or liquid can be measured by using an electrical method that directly measures energy flow into the body Using a calorimeter and a heating element you can connect the heating element to a potential difference with a recorded voltage
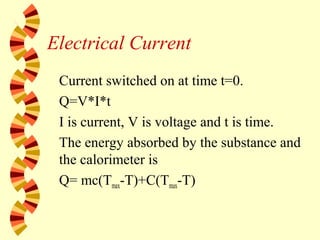
- 23. Electrical Current Current switched on at time t=0. Q=V*I*t I is current, V is voltage and t is time. The energy absorbed by the substance and the calorimeter is Q= mc(Tmax-T)+C(Tmax-T)
- 24. Method of Mixtures Have a substance of known mass, ms, temperature, tsiand specific heat cs in a calorimeter of temperature, tciand specific heat Cc. To find the specific heat of an unknown substance with known temperature tiand mass m. m*c*(ti-tf)=mscs(tf-tsi)+Cc(tf-ti)
- 25. Measuring specific latent heats To measure specific latent heat such as ice • Measure the mass of a quantity of ice • Put the ice in water that is a few degrees above room temperature • Put the ice into the water and wait until it reaches equilibrium • The energy lost by the water and calorimeter is the energy used to melt the ice and heat it up
- 26. Example Suppose 25g of ice at 0 C is placed in an aluminum calorimeter of mas 250 g (c=910) containing 300g (c=4198) of water at 24 C. The temperature of the water equilibrates at 17 C. Find the latent heat of fusion. L=340kJ/kg
- 27. Evaporation Molecules in a gas and liquid move about with a distribution of speeds If the most energetic particles are at the top of the liquid they may escape the liquid • This is known as evaporation • Different from boiling Since molecules are escaping the overall kinetic energy of the liquid is decreased • A reduced temperature of the liquid
- 28. Vapor Pressure If the liquid is in an enclosed space, the particles that evaporate collect above the liquid in what is called vapor As the liquid evaporates, the vapor pressure increases When the number of particles escaping the liquid equals the number of particles falling back into the liquid, the system has reached equilibrium.
- 29. Kinetic Theory of Gases 1. A gas consists of a large number of molecules 2. Molecules move with a range of speeds 3. The volume of the molecules is negligible compared with the volume of the gas itself 4. The collisions of the molecules with each other and the container walls are elastic 5. Molecules exert no forces on each other or the container except when in contact 6. The duration of the collisions is very small compared with the time between collisions 7. The molecules obey Newton’s laws of mechanics.
- 30. Boltzmann Equation Boltzmann Equation • The average kinetic energy of the particles is proportional to the absolute temperature of a gas. • v is the square root of the average of the squares of the speed of the molecules of the gas • If v we found the average of the speeds, it would equal 0. • v is called the root mean square speed (rms speed) • kB-Boltzmann Constant • k=1.38x10-23 J/K
- 31. Example 6 Four molecules have speed 300m/s, 350m/s, 380m/s and 500m/s. Find the average speed and the root mean square. average= 382.5m/s Rms speed= 389.5m/s It is important to note the rms speed is not exactly the average speed but a close/usable approximation
- 32. Example 7 If the root mean square molecular speed is doubled, what is the new temperature? 4x as large
- 33. Molecular explanation of Pressure Pressure is the normal force per unit area. The pressure in a gas results from the collisions of the gas molecules with the walls of its container (and not from the collisions between the molecules)
- 34. Molecular explanation of Pressure What two factors would affect the pressure of a gas? • The speed of the particles • High kinetic energy means a greater force is applied to the walls when the come in contact with the molecules (temperature) • The frequency of collisions • More collisions means more chances that the wall will get hit with a particle
- 35. Example 8 A gas is compressed slowly by a piston. Explain why the temperature of the gas will stay the same? If gas is compressed slowly by a piston then the speeds of the particles before and after the collision of the piston’s wall will be roughly equal. Since the average kinetic energy of the particles would remain roughly equal so would temperature.
- 36. Example 9 A gas is compressed rapidly by a piston. Explain why the temperature of the gas will increase. The molecules rebounding off the rapidly moving piston wall will be rebounded with greater speed. The greater speed of the molecules creates a larger kinetic energy and thus a larger temperature.
- 37. Example 10 A gas expands isothermally (temp. is constant). Explain why the pressure decreases. The volume of the gas expands, which means that, on average, molecules have a larger distance to travel between successive collisions with the walls. Thus, the collisions are less frequent than before and so the pressure decreases.
- 38. Example 11 A gas is heated at a constant pressure. Explain why the volume must increase as well. The temperature increases and so the molecules move faster, on average. An increase in temperature means the frequency of collisions must drop in order to keep pressure constant. In order for this to happen, the volume must expand to increase distance between collisions.
- 39. Ideal Gases
- 40. Pressure Pressure is defined as the normal force to an area per unit area. P=F/A SI unit • N/m2 -Pa pascal Atmospheric Pressure • 1atm=1.013x105 Pa
- 41. Example 1 Two hollow cubes of side 0.25cm with one face missing are placed together at the missing face. The air inside the solid formed is pumped out. What force is necessary to separate the cubes? 6.33x103 N
- 42. Gases It is more useful to talk about the number of moles of gas rather than the mass of the gas itself. NA= 6.02x1023 molecules/mole n=N/NA • n is the number of moles • N is the number of molecules in a gas From this we can related P, V, n, and T.
- 43. Example 2 How many molecules are there in 6 g of hydrogen gas? 1.81x1024
- 44. Example 3 Make a rough estimate of the number of water molecules in an ordinary glass of water. (.3L) 1025 molecules
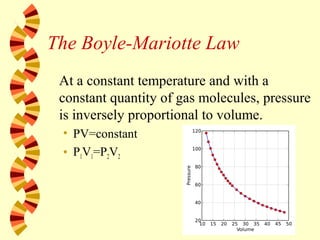
- 45. The Boyle-Mariotte Law At a constant temperature and with a constant quantity of gas molecules, pressure is inversely proportional to volume. • PV=constant • P1V1=P2V2
- 46. Example 4 The pressure of a gas is 2 atm and its volume 0.9L. If the pressure is increased to 6 atm at constant temperature, what is the new volume? 0.3L
- 47. The volume Temperature Law When the temperature is expressed in kelvin, and pressure is held constant • V/T=Constant • V1/T1=V2/T2
- 48. Example 5 A gas expands at constant pressure from an original volume of 2L at 22o C to a volume of 4L. What is the new temperature? 590K or 317o C
- 49. Pressure Temperature Law At a constant volume, pressure and temperature are proportional. • P/T= Constant P1/T1=P2/T2
- 50. Example 6 A gas in a container of fixed volume is heated from a temperature of 20o C and pressure 3 atm to a temperature of 85o C. What is the new pressure? 3.67atm
- 51. The Equation of State If we recombine the results of the previous law’s we find that • PV/T=constant Microscopically we found • PV/T=n*Constant • We found that all gases follow these rules so • PV/nT=Constant • Constant is R=8.31 J/K*mole • PV=nRT
- 52. Example 7 How many moles of gas are there in a gas of temperature 300K, volume 0.02m3 and pressure 2x105 Pa? 1.60moles
- 53. Example 8 A container of hydrogen of volume 0.1m3 and temperature of 25o C contains 3.20x1023 molecules. What is the pressure in the container? 1.3x104 Pa
- 54. Example 9 A gas of volume 2L, pressure 3 atm and temperature 300k expands to a volume of 3L and a pressure of 4 atm. What is the new temperature of the gas? 600k
- 55. Example 10 The graph below shows three isothermal curves for the same quantity of gas. Which is a the highest temperature. T1
- 57. Internal Energy Chapter 3.1 defined the internal energy of a gas as the total kinetic energy of the molecules of the gas plus the potential energy associated with intermolecular forces Ideal gas the intermolecular forces are 0
- 58. Internal Energy Ek= kB=R/NA=1.38x10-23 J/K Boltzmann Constant The Boltzmann constant is the value of the gas constant of an idea gas per molecule. The internal energy of a system can then be given by U=3/2NkT
- 59. Systems Systems are the complete set of objects that we are taking into consideration • Systems can be as large such as planet earth of the universe or small such as a cell or an atom. An closed system does not allow mass to enter or leave An isolated system does not allow energy to leave the system
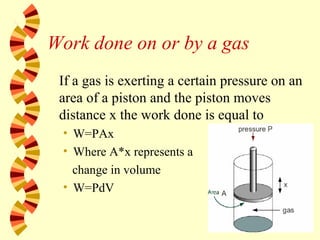
- 60. Work done on or by a gas If a gas is exerting a certain pressure on an area of a piston and the piston moves distance x the work done is equal to • W=PAx • Where A*x represents a change in volume • W=PdV
- 61. Work done on or by a gas Note that as the volume changes the pressure will also change. Thus calculus would be needed and an integral would be needed
- 62. Work done on or by a gas The total work done when the gas expands by an arbitrary amount is the area under the graph in the pressure-volume diagram
- 63. Work done on or by a gas Figure 4.4 shows an arbitrary series of changes on an idea gas that begin and end in state A. The net work done is the work done by the gas minus the work done on the gas (Figure 4.4c)
- 64. Work done on or by a gas Many processes can be identified on a pressure-volume diagram Isobaric- Pressure Isochoric-Volume Isothermal-Temp Adiabatic-Energy
- 65. Example 1 A gas is compressed at constant pressure 2.00x105 Pa from a volume of 2.00m3 to a volume of 0.500m3 . What is the work done? If the temperature initially was 40o C what is the final temperature of the gas? 3.00x105 J -195O C
- 66. Adiabatic vs Isothermal process Isothermal process means that the temperature of the gas is held constant • Changing the volume can change pressure and temperature • Isothermal keeps a constant temperature therefore constant internal energy ΔU=0 • Typically slow volume change or object is in equilibrium with its environment • Heat/cool reservoir
- 67. Adiabatic vs Isothermal process Adiabatic means that there is no thermal energy given off or absorbed by the system. • Q=0 • Typically well insulated • Fast reactions • Temperature of gas is able to change but no thermal energy can be added or subtracted ΔQ=0
- 68. The first law of Thermodynamics The change of internal energy of a system is equal to the heat supplied to the system minus the work done by the system on it’s surroundings. ΔU=Q-W U=Internal energy Q=Exchange of Heat W=Work
- 69. Example 2 A gas in a container with a piston expands isothermally (ie: the temperature stays constant). If thermal energy Q=105 J is given to the gas, what is the work done by the gas? 105 J
- 70. Example 3 A gas expands adiabatically (ie. It does not receive or lose thermal energy). Will it’s temperature increase or decrease. Decrease
- 71. Question 4 An ideal gas, kept at constant pressure 3.00x106 Pa, has initial volume 0.100m3 and temperature 300k. If the gas is compressed at constant pressure done to a volume of 0.080m3 , find: • A) the work done on the gas; • B) the thermal energy taken out of the gas • A)6.00x104 J • B) -1.5x105 J
- 72. Question 5 Figure 4.8 is a pressure-volume diagram showing two adiabatic, an isochoric and an isobaric process making up a loop ABCD for an idea gas. A)Along which legs is thermal energy supplied or removed from the gas. B) What is the relation between Qin, Qout and the work done? A) AB, CD B)W=Qin-Qout
- 73. The Second Law of Thermodynamics There are a many processes in thermodynamics that are consistent with the first law but are virtually impossible • The spontaneous flow of thermal energy from a cold body to a hotter body • The air in a room suddenly occupying just one half of the room and leaving the other half empty • A glass of water at room temperature suddenly freezing, causing the temperature of the room to rise.
- 74. The Second Law of Thermodynamics These processes do not happen because they are forbidden by a very special law of physics- The second law of thermodynamics The second law of thermodynamics deals with the limitations imposed on heat engines, devices whose aim is to convert thermal energy (disordered energy) into mechanical energy (ordered energy)
- 75. Entropy Entropy, S, is a measure of “disorder” • A measure of the number of ways a system can be rearranged The entropy of a system is not important but rather the change of entropy is important/calculable ΔS=ΔQ/T • S=entropy • Q=thermal energy supplied • T= temperature of system
- 76. The second law of Thermodynamics During a thermodynamic process the sum of the entropies of the participating systems must increase The entropy of an isolated system never decreases It is impossible for thermal energy to spontaneously flow from a cold to a hot object
- 77. Degradation of Energy It is a consequence of the second law that energy, while always being conserved, becomes less useful • It goes from ordered energy to disordered This is called energy degradation