Amalgam as restorative material lecture UG
- 1. DENTAL AMALGAM AS A RESTORATIVE MATERIAL
- 2. CONTENTS • Introduction • History • Classification • Generations of amalgam • Supplied as • Constituents in amalgam • Function of contituents • Composition of amalgam alloys
- 3. • Properties of amalgam • Manufacture of alloy powder • Indications of amalgam restoration • Contraindications of amalgam restoration • Biocompatibility of amalgam • Requirements for amalgam restoration • Setting reaction • Microstructure of set amalgam
- 4. • Manipulation of amalgam • Failures of amalgam restoration • Disadvantages of amalgam • Mercury toxicity
- 5. • ADA specification no. 1 (ISO 1559) • Word “amalgam” is derived from greek word “Emollient” which means “paste”. INTRODUCTION
- 6. • Dental amalgam is an alloy made by mixing mercury with a silver tin alloy. Dental amalgam alloy is a silver tin alloy to which varying amount of copper and small amount of zinc has been added. • According to Skinner’s, amalgam is a special type of alloy in which one of its constituent is mercury. In dentistry, it is common to use the term amalgam to mean dental amalgam.
- 7. • Dental amalgam – An alloy of silver copper and tin that is formulated and processed in the form of powder particles or compressed pellets. - Phillips’ Science of dental materials • Dental amalgam is one of the most versatile restorative materials used in dentistry. It constitutes approximately 75% of all restorative materials used by dentists. It has served as a dental restoration for more than 165 years.
- 8. • An amalgam is defined as a special type of alloy in which mercury is one of the components. Mercury is able to react with certain alloys to form a plastic mass, which is conveniently packed into a prepared cavity in a tooth. This plastic mass hardens and is stronger than any dental cement or anterior filling material. Dental amalgam is the most widely used filling material for posterior teeth. - Basic Dental Materials by John Manappalli
- 9. HISTORY • Amalgam -- First used by Chinese. There is a mention of silver mercury paste by Sukung (659AD) in the Chinese medicine and later by Li schichan • First use of room temp. mixed amalgam- Bell in England 1819 (Bell’s putty) • Traveau in France (1826) – advocated a mixture of silver and. mercury as a filling material – produced amalgam by grinding silver coins with mercury.
- 10. • 1833 – Introduction of Royal Mineral Succedaneum to USA as substitute for gold – Crawcour Brothers- “Royal mineral succedaneum” • 1840s– AMALGAM WAR • 1859- ADA was formed • 1860’S -1870’S – Elisa townsend and Flagg did a lot of work to improve Dental Amalgam
- 11. • 1895- To overcome expansion problems G.V. Black developed a formula for modern amalgam alloy 67% silver, 27% tin, 5% copper, 1% zinc • 1920 – Dr Grey – Delayed expansion • 1926 - Second amalgam war – Europe – as a result of the writings of Alfred Stock, a professor of Chemistry published papers on the dangers of mercury vapor
- 12. • 1937- Gaylerin found that in the coarse filling alloys of that time, copper contents greater than 6% produced excessive expansion • This was later challenged by Greener in 1970’S • 1946 - Skinner, added copper to the amalgam alloy composition in a small amount. This served to increase strength and decrease flow
- 13. • 1959 – Dr. Wilmer Eames recommended a 1:1 ratio of mercury to alloy, thus lowering the 8:5 ratio of mercury to alloy that others had recommended. • 1962- spherical particle dental alloy was introduced by Innes & Youdelis • 1963 – Innes and Youdelis – High Cu admixed alloy • 1970- Change from hand trituration to mechanical trituration
- 14. • 1973 - Current controversy – termed Third amalgam war – due to writings of Dr HA Higgins • 1973 - First single composition spherical alloy named Tytin (Kerr) a ternary system (silver/tin/copper) was discovered by Kamal Asgar of the University of Michigan • May 1991- Illinois house of representatives concluded amalgam was safe. • August 1991- national institute of health technology concluded amalgam was safe.
- 15. 1.According to number of alloy metals: • Binary alloys (Silver-Tin) • Ternary alloys (Silver-Tin-Copper) • Quaternary alloys (Silver-Tin-Copper-Indium) 2. According to whether the powder consist of unmixed or admixed alloys: Certain amalgam powders are only made of one alloy. Others have one or more alloys or metals physically added (blended) to the basic alloy. E.g. Adding copper to a basic binary silver tin alloy CLASSIFICATION
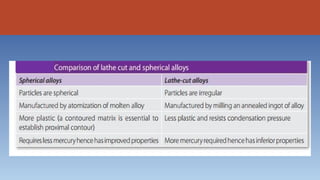
- 16. 3. According to the shape of the powdered particles: • Spherical shape (smooth surfaced spheres) • Lathe cut (Irregular ranging from spindles to shavings) • Combination of spherical and lathe cut (admixed)
- 17. 4. According to Powder particle size: • Micro cut • Fine cut • Coarse cut 5. According to copper content of powder : • Low copper content alloy - Less than 4% • High copper content alloy (more than 10%) - a) Admixed alloy b) Single-composition alloy
- 18. 6. According to Presence of zinc: • Zinc containing (more than 0.01%) • Non - zinc containing (less than 0.01%)
- 19. GENERATIONS OF AMALGAM Class–I Silver and tin in ratio (8:1) Class–II Silver, tin, copper (4%), zinc Class–III Silver eutectic alloy added to original alloy Class–IV Copper content increased to 29% • Class–V Indium added to mixture of silver, tin and copper • Class–VI Noble metals added such as palladium
- 20. SUPPLIED AS • Bulk powder and mercury in separate containers. • • Alloy and mercury in disposable capsules. • Preweighed alloy as tablet form in tubes.
- 21. CONSTITUENTS IN AMALGAM Basic Constituents : • Silver • Tin • Copper • Mercury Other Constituents : • Zinc • Indium and Pallidum
- 22. Silver (Ag) - • increases strengh • increases expansion Tin (Sn) - • decreases expansion • decreases strength • increases setting time FUNCTION OF CONSTITUENTS
- 23. Copper (Cu) - • ties up tin(reducing gamma-2 formation) • increases strength • reduces tarnish and corrosion • reduces creep (reduces marginal deterioration)
- 24. Mercury (Hg)- • activates reaction • only pure metal that is liquid at room temperature • spherical alloys (require less mercury, smaller surface area, easier to wet 40% to 45% Hg) • admixed alloys (require more mercury lathe-cut particles more difficult to wet 45% to 50% Hg)
- 25. Zinc (Zn)- • used in manufacturing • decreases oxidation of other elements, acts as sacrificial anode • provides better clinical performance (less marginal breakdown) • causes delayed expansion with low Cu alloys (if contaminated with moisture during condensation)
- 26. Indium (In)- • decreases surface tension (reduces amount of mercury necessary, reduces emitted mercury vapor) • reduces creep and marginal breakdown • increases strength • must be used in admixed alloys
- 27. Palladium (Pd)- • reduced corrosion • greater luster Platinum(Pt)- • Hardens the alloy and increases resistance to corrosion
- 28. COMPOSITION OF AMALGAM ALLOYS
- 29. PROPERTIES OF AMALGAM • Microleakage Penetration of fluids and debris around the margins may cause secondary caries. Dental amalgam has an exceptionally fine record of clinical performance because of its tendency to minimize marginal leakage. Self sealing The small amount of leakage under amalgam restorations is unique. If the restoration is properly inserted, leakage decreases as the restoration ages in the mouth.
- 30. This may be due to the formation of corrosion products in the tooth- restoration interface. Over a period of time they seal the interface and reduce leakage. Thus amalgam is a self sealing restoration. • Dimensional Changes The earliest amalgams exhibited expansion while setting. This was because of the greater mercury/alloy ratio used. Amalgams may expand or contract, depending on its manipulation.
- 31. Ideally, dimensional change should be small. Excessive contraction can lead to microleakage, sensitivity and secondary caries. Excessive expansion can produce pressure on the pulp and postoperative sensitivity. Protrusion of the restoration can also occur. ISO Sp. 24234:2015 requires that amalgam should not expand more than 0.15% or contract less than –0.1% at 37 °C, during hardening. Mechanically, triturated modern amalgams, both low and high copper, prepared from low mercury/alloy ratios show a slight contraction.
- 32. • Effect of moisture contamination If a zinc-containing-low-copper or high-copper amalgam is contaminated by moisture during trituration or condensation, a large expansion can take place. It usually starts after 3-5 days and may continue for months, reaching values greater than 400 µm (4%). This is known as delayed expansion or secondary expansion. The expansion is caused by the releases of hydrogen gas from the reaction of zinc with water.
- 34. • Compressive strength Well designed amalgam restorations have sufficient compressive strength to withstand normal intraoral masticatory forces.
- 35. • Tensile strength Amalgam cannot withstand high tensile or bending stresses and can fracture easily in improperly designed restorations. Therefore, the cavity should be designed so that the restoration will receive minimal tension or shear forces in service. For Low copper alloys – 60MPa Admixed – 48MPa Single composition – 64MPa
- 36. • Creep It is defined as a time dependent plastic deformation. Creep of dental amalgam is a slow progressive permanent deformation of set amalgam, which occurs under constant stress (static creep) or intermittent stress (dynamic creep). Creep is related to marginal breakdown of low-copper amalgams. The higher the creep, the greater is the degree of marginal deterioration.
- 37. According to ISO 24234:2015 creep should be below 2%
- 38. • Tarnish and corrosion Amalgam restorations often tarnish and corrode in the mouth. Black silver sulfide can form on the surface of an amalgam restoration in some patients. Both high and low-copper amalgams show corrosion. However corrosion in high-copper amalgams is limited because η phase is less susceptible.
- 39. MANUFACTURE OF ALLOY POWDER The various components of the amalgam alloy are combined together by melting to form ingots. The ingots have to be heat- treated in an oven for a set period of time. This process is called annealing. Annealing improves the homogeneity and grain structure of the alloy. • Lathe-Cut Alloy Powder An annealed ingot of silver-tin alloy is placed in a lathe and fed into a cutting tool. The resulting chips obtained are often needle like and some manufacturers reduce the chip size.
- 40. • Aging, acid treatment and annealing of particles A freshly cut alloy reacts too rapidly with mercury. If the alloy filings are stored at room temperature for a few months, the reactivity gradually decreases. Such alloys are said to have been aged. The filings can be aged faster by boiling in water for 30 minutes. Aging also improves the shelf life of the product. Some manufacturers treat the filings with acid to improve reactivity.The stresses induced during the cutting and grinding process must be relieved by an annealing.
- 41. • Spherical Alloy Powder The spherical alloy is prepared by an atomization process. The liquid alloy is sprayed under high pressure of an inert gas through a fine crack into a large chamber. If the droplets solidify before hitting a surface, the spherical shape is preserved. Like the lathe-cut powders, spherical powders are aged.
- 44. INDICATIONS OF AMALGAM RESTORATION 1. Moderate to large class I preparation. 2. Class II preparations in which there is: • Heavy occlusion • Extension on the root surface • Problem of isolation. It is indicated in heavy occlusion because amalgam has greater wear resistance than composites. Minor contamination during the amalgam placement has less adverse effects as compared to composite restorations.
- 45. 3. Class V preparations in which: • Aesthetic is not a problem • Preparation entirely on root surface • Isolation is difficult. 4. Class VI preparations. 5. Class III preparations (sometimes) where isolation is difficult. 6. Used as a foundation in cases of grossly decayed teeth while planning for cast restoration.
- 46. 7. Used as a postendodontic restoration. 8. Teeth having nondefinitive pulpal prognosis—used as a type of interim restoration before assessment of pulpal status of the tooth. 9. Tooth having fractured cusp can be restored with the help of amalgam using pin and slot.
- 47. CONTRAINDICATIONS OF AMALGAM RESTORATION 1. Esthetics: Use of amalgam is avoided in aesthetic areas of oral cavity. So, preparations class III, IV, V usually are not indicated except in certain cases. 2. Small to moderate class I and class II preparations should be restored with composite rather than amalgam as former results in more conservative tooth preparation. 3. Amalgams are contraindicated in patients who are allergic to alloy components.
- 48. BIOCOMPATIBILITY OF AMALGAM • Dental amalgam is prepared easily. • It is relatively inexpensive compared with most other restorative materials. • The longevity of dental amalgam restorations is high. • The manipulation of dental amalgam after placement in the prepared tooth cavity is easy. • The material has low creep, high compressive strength, and high resistance to wear. • It experiences minimal dimensional change with time.
- 49. • There is no other dental material known for marginal-sealing capacity that occurs with time after the restoration is placed in the oral cavity, which results in low microleakage. • The clinical placement of a dental amalgam restoration is not as technique-sensitive to operating conditions, such as the presence of saliva in the oral environment. • Sealing ability improves with age by formation of corrosion products at tooth amalgam interface. • Minimal postoperative sensitivity
- 50. REQUIREMENTS FOR AMALGAM RESTORATION The required tooth preparation form must allow the amalgam to - • possess a uniform specfied minimum thickness for strength (so that it will not lex and fracture under load. • produce a 90-degree amalgam angle (butt-joint form for maximum edge thickness) at the margin. • be mechanically retained in the tooth
- 51. SETTING REACTION • Low Copper Amalgam – When alloy powder and mercury are triturated, the silver and tin in the outer portion of the particles dissolve into the mercury. Simultaneously, the mercury diffuses into the alloy particles and starts reacting with the silver and tin within forming crystals of silver -mercury (Ag2Hg3) and tin-mercury compounds (Sn8Hg)
- 52. Silver-tin compound (unreacted alloy powder) is known as the gamma(γ) phase. The silver mercury compound is known as gamma 1 (γ1) phase and the tin-mercury as the gamma 2 (γ2) phase.
- 53. • Admixed Alloys When the components are mixed the mercury begins to dissolve the outer portion of the particles. Silver from the silver-copper eutectic alloy particles, and both silver and tin from the silver-tin alloy particles enter the mercury. The tin dissolved in the mercury reacts with the copper of the silver-copper particles and forms the Cu6Sn5 (η or Eta). The η crystals form around the unreacted silver-copper particle.
- 54. • At the same time γ1 phase is also formed. As in the low copper alloys γ1 surrounds everything forming the matrix. γ2 is also formed at the same time but is later replaced by η. Thus in admixed alloy the undesirable γ2 phase is greatly reduced.
- 55. • Single Composition Alloy Though each particle has the same composition, the silver, tin and copper present exist in various phases within the particle. Thus each particle contains Ag3Sn (γ), AgSn (β) and Cu3Sn(ε ). When triturated, silver and tin from the particle dissolve in mercury forming the γ1 (Ag2-Hg3) crystal matrix that binds together the partially dissolved alloy particles. At this stage very little copper dissolves.
- 56. Later, a layer of η (Cu6Sn5) crystals are formed at the surface of alloy particles. Some η (Cu6Sn5) crystals also form in the matrix.
- 59. MANIPULATION OF AMALGAM • Selection of alloy and mercury • Mercury alloy ratio (Proportioning) • Trituration (Mechanical and hand) • Mulling • Condensation (Hand and mechanical) • Pre-carve burnishing • Carving • Post-carve burnishing • Finishing and polishing
- 60. • Selection of amalgam alloys Following factors are considered while selecting an alloy for restoration: a. Type of alloy: 1. High copper or low copper alloys 2. Zinc-free or zinc-containing alloys 3. Size and shape of the particles
- 61. b. If restoration undergoes high occlusal stresses, choose amalgam with high resistance to marginal fracture. c. Patients with psychological problems or other diseases, requiring early disposal, indicate the use of fast setting alloy. d. In wider and broader preparations, alloy with low creep values is preferred.
- 62. • Mercury Alloy Ratio (Proportioning) For success of the restoration, mercury ratio should be specific and accurate according to type of alloy used.Mercury is basically required to wet the alloy particles before they can react. Eames has preferred 1:1 ratio of alloy/mercury for best results. Generally, it is 5 : 8 or 5 : 7, if mercury content is more than required amount, resultant mix will be weaker, but if it is less, it might not sufficiently wet the alloy particles. Lathe-cut amalgam alloys require more (45%) of mercury to wet than the spherical alloys (40%)
- 64. • Trituration (Mechanical and hand) The purpose of trituration is to remove oxide layers from the alloy particles so as to coat each alloy particle with mercury, resulting in a homogeneous mass for condensation.Trituration can be done by hand or mechanical means. Mechanical method is done with the help of automatic amalgamator and hand method of trituration is done with the help of mortar and pestle.
- 66. • Effect of trituration on strength depends on the type of amalgam • alloy, the trituration time and the speed of the amalgamator. • Either, under trituration or over-trituration decreases the strength for • both traditional and high copper amalgams. • More the trituration energy used, more evenly distributed are the • matrix crystals over the amalgam mix and consequently more the • strength pattern in the restoration. • Excess trituration after formation of matrix crystals will create cracks • in the crystals, lead to drop in strength of set amalgam.
- 67. Over-trituration: Alloy will be hot, hard to remove from the capsule, shiny wet and soft. Under-trituration: Alloy will be dry, dull and crumbly; will crumble if dropped from approx 30 cm.
- 68. Normal Mix: Shiny appearance separates in a single mass from the capsule.
- 69. • Mulling Mulling is actually a continuation of trituration. It is done to improve the homogeneity of the mass and get a single consistent mix. It can be accomplished in two ways - 1. The mix is enveloped in a dry piece of rubber dam and vigorously rubbed between the first finger and thumb, or the thumb of one hand and palm of another hand for 2–5 seconds.
- 70. 2. After trituration the pestle is removed and the mix is triturated in the pestle-free capsule for 2–3 seconds. Mulling is not required for mechanical triturated amalgams.
- 71. • Condensation (Hand and mechanical) The amalgam is placed in the cavity after trituration, and packed (condensed) using suitable instruments. Aims - 1. To compact the mass to increase the density of the restoration. 2. To reduce voids. 3. To remove excess mercury. 4. To adapt the amalgam to the preparation walls and margins.
- 72. Rules of Condensation - 1. Start condensation within three minutes of trituration. 2. Condense continuously. 3. Condense laterally as well as apically. 4. Apply adequate force for condensation. 5. Have a constant supply of amalgam.
- 74. • Pre-carve burnishing Precarve burnishing is done after condensation. It is the process of rubbing, generally done to make the surface shiny. Amalgam is overfilled and burnished immediately with heavy strokes so as to improve marginal adaptability of the restoration and remove excess mercury from overpacked amalgam
- 75. Advantages of Precarve Burnishing – 1. Improves the marginal integrity of . restoration. 2. Shapes the restoration according to contours and curvatures of the tooth. 3. Helps in reducing the mercuric content of amalgam.
- 76. • Post- carve burnishing It is done after completion of carving with the help of small sized burnishers using light strokes. Advantages of Postcarve Burnishing 1. Reduces number of voids on surface of restoration 2. Produces denser amalgam at margins.
- 77. 3. Improves marginal seal. 4. Increases surface hardness. 5. Decreases rate of corrosion.
- 79. • Finishing and polishing Finishing amalgam restorations involves removal of marginal irregularities, defining anatomical contours, and smoothening the surface roughness of the restoration. Polishing is done to achieve a smooth, shiny luster on the surface of the amalgam. Finishing is done before polishing by use of abrasive agents that are coarse enough to remove the bulk from thesurface. Polishing requires mildly abrasive materials for producing smooth and shiny surface.
- 80. Advantages of Finishing and Polishing 1. Improves marginal adaptation of restoration by removing flash. 2. Reduces tarnish and corrosion. 3. Polished surface is plaque-resistant. 4. Polished surface is smoother and easier to clean. 5. Prevention of recurrent decay. 6. Prevention of amalgam deterioration.
- 82. FAILURES OF AMALGAM RESTORATION The reasons for failure of amalgam restorations are – 1. Poor case selection 2. Defective tooth preparation 3. Defective amalgam manipulation 4. Defective matrix adaptation 5. Postrestorative failures
- 83. DISADVANTAGES OF AMALGAM • Less aesthetic • Extensive preparation to hold an amalgam filling. • Amalgam fillings can corrode or tarnish over time,causing discoloration. • Does not bond to tooth. • Being metallic restoration it is non-insulating • Marginal degradation is seen in low copper alloys.
- 84. • Amalgam is not strong enough to reinforce the weakened tooth structure. • Poor tensile strength making it a brittle material • Results in galvanic current in association with gold restoration or even in same restoration with non-uniform condensation. • Oral lichen planus is also seen with amalgam restoration.
- 85. MERCURY TOXICITY Mercury is toxic to living creatures. Free mercury should not be sprayed or exposed to the atmosphere. This hazard can arise during trituration, condensation and finishing of the restoration, and also during the removal of old restorations at high speed. Mercury vapors can be inhaled. Skin contact with mercury should be avoided as it can be absorbed.Mercury has a cumulative toxic effect. Dentists and dental assistants, are at high risk. Though it can be absorbed by the skin or by ingestion, the primary risk is from inhalation.
- 86. THANK YOU
- 87. REFERENCES • Sturdevant’s Art and Science of Operative Dentistry (edition 7th) Page no. 306-410 • Phillips Science of dental materials , (edition 12th) Page no. 476 - 477 • V shama Bhatt Science of dental materials ( edition 2nd ) Page no. 37, 470 • Preclinical manual of prosothodontic (S Lakshmi ) (3rd edition) Page no. 294 - 305 • Textbook of prosthodontics by V Rangrajan (2nd edition) Page no. 194 - 195 • Essentials of complete denture prosthodontics by • sheldon winkler (2nd edition) Page no. 132-133 • Basics of dental material by John Manappallil (4th edition) Page no. 542-543