Antibiogram CLSI Recommendations
- 1. By: Dr Mostafa Mahmoud PhD, Consultant Microbiologist, Riyadh, MOH Assist. Professor of Medical Microbiology & Immunology, Faculty of Medicine, ASU, Cairo, Egypt.
- 2. What is an Antibiogram (ABG)? An antibiogram is an overall profile of antimicrobial susceptibility testing (AST) results of a specific microorganism to a battery of antimicrobial drugs. This profile is generated by the laboratory using aggregate data from a hospital or healthcare system; data are summarized periodically and presented showing percentages (%) of organisms tested that are susceptible to a particular antimicrobial drug. Only results for antimicrobial drugs that are routinely tested and clinically useful should be presented to clinicians.
- 3. How to measure the antimicrobial susceptibility in the micro lab? 1- Disc Diffusion (DD) method and E-test (Manual labs). 2- The broth dilution (MIC) method either macro or microdilution (Automated labs).
- 4. 1- Disc Diffusion method (Manual labs) Disc Diffusion Ring Disc Diffusion E- Test.
- 5. 2- Broth Microdilution (Microscan)
- 6. 2- Broth Microdilution (Phoenix)
- 7. 2- Broth Microdilution (Vitek 2)
- 8. Recommendations for Antibiograms: Analyze/present cumulative antibiogram report at least annually; Include only final, verified test results; Include only species with testing data for ≥ 30 isolates. Include only diagnostic (not surveillance) isolates Eliminate duplicates by including only the first isolate of a species/patient/analysis period, irrespective of body site or antimicrobial profile Include only antimicrobial agents routinely tested; do not report supplemental agents selectively tested on resistant isolates only. Report %S (Susceptible) and do not include %I (intermediate) in the statistic.
- 9. Antibiogram Uses: 1- Antibiograms help guide the clinician and pharmacist in selecting the best empirical antimicrobial treatment in the event of pending microbiology culture and susceptibility results. 2- They are also useful tools for detecting and monitoring trends in antimicrobial resistance.
- 10. Scope of applications of ABG: 1- Staff working in analysis and presentation of AST data (e.g. clinical microbiologists, pharmacists, physicians). 2- Staff utilizing cumulative AST data to make clinical decisions (e.g. clinical microbiologists, infectious disease specialists and other clinicians, infection control practitioners, pharmacists, epidemiologists, other health care personnel, and public health officials). 3- For designing information systems for the storage and analysis of AST data (e.g. laboratory information system [LIS] vendors, manufacturers of diagnostic products that include epidemiology software packages).
- 11. Data Required to perform ABG: 1. Patient: - Required: ID, - Desirable: Age, Sex, Location (Ward), Admission date; 2. Specimen information: - Required: number, type & date of collection. - Desirable: Body site e.g. right or left
- 12. 3. Organism information: - Required: identification up to the genus or species level (genus can be satisfactory). - Desirable: Isolate number, change in name of isolate (if happen), Infection control data e.g. colonization or infection, community- acquired, or healthcare associated CA or HAI 4. AST information: - Required: final MIC or zone diameter (ZD) used, method used, special tests for detection of B- lactamase, mecA gene, PBP2a by agar screening , PCR or latex testing respectively. - Desirable: detailed MIC or ZD
- 13. Frequency of Performance At least once yearly. More frequent with high number of isolates, new antimicrobial introduced, or presence of important medical changes. Facility: ABG is performed based upon local institution- specific susceptibility data.
- 14. Data presentation: In a tabular form. No universal formats Separate tables for Gram-positive, and Gram- negative, also for anaerobes and yeasts if applicable. Arrange the organisms within the table alphabetically, by organism group of by the prevalence.
- 15. Some labs may present data by body site e.g. urine gram-negative or gram-positive. Antibiogram for critical care units (more resistant) e.g. ICUs better to be separated to compare %S with the total hospital. Comments upon the table must be included to explain it. Recommended species to be included (even if <30 isolates) in the tables are the following:
- 16. Gram-negative species: Acinetobacter baumannii • Citrobacter freundii Enterobacter aerogenes • Enterobacter cloacae Escherichia coli • Haemophilus influenzae Klebsiella pneumoniae • Morganella morganii Proteus mirabilis • Providencia spp. Pseudomonas aeruginosa • Salmonella spp. Serratia marcescens • Shigella spp. Stenotrophomonas maltophilia
- 17. Example for Gram-negative ABG - Comments upon the table must be included. - Denoting of abbreviations must be included e.g. AMK= Amikacin.
- 18. Gram-positive species: Enterococcus spp. (preferable to be in separate species). Staphylococcus aureus Coagulase-negative Staphylococcus spp. (exclude S. lugdunensis and S. saprophyticus, separately if in sufficient numbers). Streptococcus pneumoniae. Viridans group Streptococcus spp. (from usually sterile body sites only).
- 19. Example for Gram-Positive ABG - Comments upon the table must be included. - Denoting of abbreviations must be included e.g. CLI= Clindamycin
- 20. Example for Gram-Positive ABG
- 21. Example for Gram-Positive ABG
- 22. Anaerobes Bacteroides fragilis Clostridium perfringens
- 23. Example of ABG data Report by alphabetically listing of Antimicrobial Agents.
- 24. Example of Antibiogram data Report by class of Antimicrobial Agents.
- 25. Presenting Emerging Resistance Trends: Presentation of resistant organisms over years can be presented in tables or graphs e.g.: MRSA (S. aureus – %S for oxacillin - inpatient and outpatient); VRE (Enterococcus spp. – %S to vancomycin isolates from sterile body sites); E. coli – %S to trimethoprim-sulfamethoxazole (urine isolates) and fluoroquinolones; ESBLs (K. pneumoniae and E. coli – % of isolates that produce ESBLs); P. aeruginosa – %S to fluoroquinolones and/or imipenem.
- 26. Presenting trends in resistance to Oxacillin (MRSA):
- 27. Different ABG presentation formats By nursing unit or care sits: i.e. patient location e.g. ICU, Burn unit, Ward, OPD, nursing home for the better selection of site specific empirical therapy. By organism’s resistance character: especial for MDROs e.g. MRSA, VRE, MDR Acineto., ESBLs. By specimen type or infection site: e.g. urine isolates, blood isolates). By clinical service or patient population: to guide initial empirical antimicrobial therapy for specific patient types (e.g. surgical, pediatric, cystic fibrosis, transplant, burn).
- 28. Data presentation in tables: 1- Date of the period of the Report. 2- Name of the Laboratory of the facility. 3- Comments upon methodology used in preparation of data. 4- Organism name and numbers
- 29. Calculations Report the first isolates of pathological organisms only not surveillance one. Only report organism with more than 30 isolates. Use CLSI recommended antimicrobials to each species. Report only the percentage of susceptible (S%) not intermediate. Give in the form of tables and graphs.
- 30. Number of isolates: Typically should > 30 isolates to be statistically significant. If less than 30 you have to put comment upon the table. You can group similar species together to increase the numbers e.g. salmonella or shigella species.
- 31. Distribution Formats: In easy accessible formats to all prescribing Physicians, Infection control staffs, Pharmacists, Epidemiologists & Microbiology Staffs‘:- Small cards in coat pocket Or in laminated sheets. In the institution website (Intranet). As application or PDF. Printed formats especially in special areas like ICUs.
- 34. Antibiogram laminated sheet GP & Anaer.
- 35. Antibiogram laminated sheet GN
- 36. Methods of calculations: 1- Confidence Intervals (CI): it gives the precision of susceptibility % and depends upon the numbers of isolates. 2- Statistical Significance of Changes in Susceptibility Rates: use Chi-square test to compare different S% in different years. A P value of ≤ 0.05 is accepted for being significantly different.
- 37. Limitations of Data, Data Analysis, and Data Presentation Culturing Practices: sample collection, transport and storage. Bias in treatment e.g. in OPD, change in culturing technique in the lab. Influence of Small Numbers of Isolates: number of isolates to be > 30.
- 38. Antibiogram Limitations: 1. Minimum inhibitory concentrations (MICs) are not included; as a result subtle trends below the resistance threshold (known as “MIC creep”) are not reflected. 2. Data do not take into account patient factors such as history of infection or past antimicrobial use. Resistance patterns for certain drugs vary significantly by age, and a patient’s underlying medical condition may affect how well an antimicrobial works
- 39. 3. Data are the result of single organism- antimicrobial combinations, therefore do not show trends in cross-resistance of an organism to other drugs, nor do they reveal synergistic properties of antimicrobials used in combination. 4. Data may not be generalizable to specific patient populations or locations of a healthcare facility if the antibiogram is compiled using hospital- or healthcare system-wide data.
- 40. Examples of Accepted Antibiogram (KSH)
- 41. KSH
- 42. KKHK
- 43. Iman
- 44. (Iman)
- 46. No Comments!!!!!
- 48. Graphs not from our hospitals Klebsiella - Percentage of sensitive Isolates (178). 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 80% 37.60% 29.20% 37.10% 34.30% 61.20% 35.40% 32% 43.30% 75.30% 54.50% 87.60% 62.40% 83.70% 30.30% 68.50%68.50% 39.30% 29.30% Series1
- 49. Pseudomonas Aeruginosa - Percentage of sensitive Isolates (127). 0.00% 10.00% 20.00% 30.00% 40.00% 50.00% 60.00% 70.00% AKCEFPCTXCAZCIPFOSFGNIPMLEVOMEROPIPTAZTOB 60.60% 48.80% 3.90% 48% 52.80% 38.60% 52.80% 55.90% 54.30% 58.30% 55.90% 66.90% 54.30% Series1
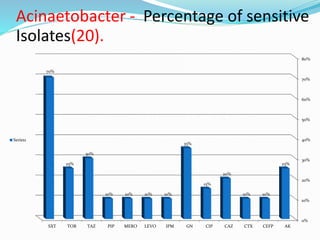
- 50. Acinaetobacter - Percentage of sensitive Isolates(20). 0% 10% 20% 30% 40% 50% 60% 70% 80% AKCEFPCTXCAZCIPGNIPMLEVOMEROPIPTAZTOBSXT 25% 10%10% 20% 15% 35% 10%10%10%10% 30% 25% 70% Series1
- 51. Staph.aureus - Percentage of sensitive Isolates (67). 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%97% 3% 97% 89.60% 70% 95.50% 98.50% 91% 95.50% 100%100% 3% 100%100%100% 98.50% 100% Series1
- 54. References CLSI. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Approved Guideline—Third Edition. CLSI document M39-A3. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. King Fahd Medical City Antibiograms. King Khaled Hospital Hafr Al-Baten
- 55. Thank you










![Scope of applications of ABG:
1- Staff working in analysis and presentation of AST data
(e.g. clinical microbiologists, pharmacists, physicians).
2- Staff utilizing cumulative AST data to make clinical
decisions (e.g. clinical microbiologists, infectious
disease specialists and other clinicians, infection
control practitioners, pharmacists, epidemiologists,
other health care personnel, and public health
officials).
3- For designing information systems for the storage and
analysis of AST data (e.g. laboratory information system
[LIS] vendors, manufacturers of diagnostic products
that include epidemiology software packages).](https://image.slidesharecdn.com/antibiogrammypresention-170404064431/85/Antibiogram-CLSI-Recommendations-10-320.jpg)