Causal Inference from Pragmatic Trials.
- 1. @EpiEllie Causal Inference from Pragmatic Trials Eleanor Murray, ScD Department of Epidemiology University of Michigan May 8, 2019
- 2. Acknowledgements Collaborators Ellen Caniglia, Sonja Swanson, Sonia Hernández- Díaz, and Miguel Hernán Funding This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Award (ME- 1503-28119) All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient- Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee. Murray. 2019. Pragmatic randomized 2
- 3. Outline I. What are pragmatic trials? II. What are patient-centered causal effects? III. Can we estimate per-protocol effects? IV. Some guidelines for trial design & analysis 3Murray. 2019. Pragmatic randomized
- 4. Part I: What are pragmatic trials?
- 5. optimal for detecting treatment effects Randomization prevents confounding … But, results can have limited applicability for clinical decision- making highly selected population short duration & intermediate or surrogate outcomes comparators not clinically relevant 5Murray. 2019. Pragmatic randomized
- 6. Solution: Pragmatic randomized trials Definition: A randomized trial designed to assess real-world effectiveness of interventions Pragmatic randomized trials are designed to ask clinically relevant, generalizable, questions 6Murray. 2019. Pragmatic randomized
- 7. Pragmatic randomized trials https://www.gopubmed.org/web/gopubmed/statistics/”Pragmatic+Clinical+Trials +as+Topic”[mesh] 7Murray. 2019. Pragmatic randomized
- 8. Trade-off: clinical relevance vs bias Long duration +Allows a more relevant primary outcome –Increases chances of loss to follow- up –Increases chances of non- adherence Usual care as comparison +Answers a more clinically relevant question 8Murray. 2019. Pragmatic randomized
- 9. Part II: What are patient- centered causal effects?
- 10. Patient-centered causal effects Definition: causal effects which provide patients with usable information for shared decision-making 10Murray. 2019. Pragmatic randomized Murray EJ, et al. Journal of Clinical Epidemiology. 2018;
- 11. Intention-to-treat & stratified ITT are patient-centered Murray. 2019. Pragmatic Randomized 11 Murray et al. 2018 J Clin Trials, 103:10-21.
- 12. Non-inferiority results viewed with extreme skepticism “To me there has to be a point that they developed this drug. Like what else is going on with the drug?… It’s really not effective. And not only is it equally effective than the drug that has already been around, it’s inconvenient” Murray et al. 2018 J Clin Trials, 103:10-21. 12Murray. 2019. Pragmatic randomized
- 13. Superiority estimates are patient-centered Murray. 2019. Pragmatic Randomized 13Murray et al. 2018 J Clin Trials, 103:10-21.
- 14. Patients understand absolute measures Murray. 2019. Pragmatic Randomized 14Murray et al. 2018 J Clin Trials, 103:10-21.
- 15. Patients who expect to adhere may change their choices based on per- protocol effects “It would depend on how critical the case was. If I had serious COPD and there was, both parents had died of it, I would say, ‘You know what? I am committed to my health. I’m committed to taking it as prescribed.’ So I’d be willing to try the new [less convenient] drug.” 15Murray. 2019. Pragmatic randomized Murray et al. 2018 J Clin Trials, 103:10-21.
- 16. Per-protocol effects are patient- centered when patients expect to adhere Murray. 2019. Pragmatic Randomized 16 Murray et al. 2018 J Clin Trials, 103:10-21.
- 17. Part III: Per-protocol effects are patient-centered, but can we estimate them?
- 18. First, a closer look at the intention-to-treat effect The ITT answers the question How does the outcome change if everyone is randomized to exposure A vs exposure B? Murray. 2019. Pragmatic Randomized
- 19. Intention-to-treat effect gives an unbiased estimate of effect of randomization Randomization ensures no confounding at baseline for treatment assignment Treatment happens after randomization Loss to follow-up happens after randomization Post-randomization events are not guaranteed to be unconfounded! 19Murray. 2019. Pragmatic randomized
- 20. Effect of randomization is not really an interesting effect Often a lower bound on the effect of treatment compared to placebo Lower bound is insufficient for adverse events or safety When comparing active treatments, ITT can vary towards or away from the null No real world, clinical, equivalent of randomization 20Murray. 2019. Pragmatic randomized
- 21. Effect of treatment is an interesting effect Relevant for real world, clinical, decision making Allows better risk assessment for adverse events or safety Interpretable for both placebo and active / usual care comparators 21 Per-protocol effect is the effect we really want! Murray. 2019. Pragmatic randomized
- 22. What is the per-protocol effect? A per-protocol effect answers the question How does the outcome change if everyone is receives exposure A vs exposure B? Murray. 2019. Pragmatic Randomized
- 23. This is what we want but we can’t get it from data alone All causal effects require that we make assumptions that cannot be verified in the data Murray. 2019. Pragmatic Randomized
- 24. What assumptions do we need? 1. No unmeasured confounding: all common causes of the treatment and outcome are known and measured in the data Murray. 2019. Pragmatic Randomized
- 25. Intention-to-treat effect has no unmeasured confounding for randomization Murray. 2019. Pragmatic Randomized
- 26. always requires adjustment for confounding Murray. 2019. Pragmatic Randomized
- 27. What assumptions do we need? 2. Positivity: non-zero probability of all levels of treatment for all individuals in our target population (i.e. variability in exposure) Murray. 2019. Pragmatic Randomized
- 28. Randomization guarantees positivity for the intention-to- treat effect Murray. 2019. Pragmatic Randomized
- 29. Per-protocol effects may not have positivity Murray. 2019. Pragmatic Randomized
- 30. What assumptions do we need? 3. Consistency: our treatment levels are clear and well-defined Are we asking a specific enough question to get an answer we can understand? Murray. 2019. Pragmatic Randomized
- 31. Why are well-defined interventions important? When there are multiple possible ‘interventions’ and we don’t specify one, our answer is a weighted average of all interventions but we don’t know the weights Murray. 2019. Pragmatic Randomized
- 32. Why are well-defined interventions important? But, if the intervention is ill-defined the confounding is probably also ill- defined! Murray. 2019. Pragmatic Randomized
- 33. Recap: causal effect options 1.Intention-to-treat effects Effect of randomization to treatment 2.Per-protocol effects: effect of treatment Effect of initiating treatment Effect of adhering to treatment protocol Effect of receiving point intervention, among the ‘compliers’ (not necessarily all adherers!) 33Murray. 2019. Pragmatic randomized
- 34. But, per-protocol analyses have a bad reputation 34
- 35. What is a per-protocol analysis? Common approaches censor when non-adherent, don’t adjust for confounding add adherence to regression model, adjust only for baseline confounders 35 Common ≠ correct! Murray. 2019. Pragmatic randomized
- 36. Per-protocol analyses tell us how did trial outcomes differ between those who did adhere to, or recieved, assignment A and those who did adhere to, or receive, assignment B? 36Murray. 2019. Pragmatic randomized
- 37. Per-protocol analyses in the literature Approach Description 1. “Modified ITT” censor never initiators 2. “As-treated” allow cross-over censor non-initiators or discontinuers 3. “Per-protocol population” censor if never initiate, cross-over, or discontinuation 4. Adherence adjustment include adherence in model for outcome model 5. Instrumental variables, aka compare outcome by trial arm, and correct 37Murray. 2019. Pragmatic randomized
- 38. Per-protocol analyses in the literature Approach Description 1. “Modified ITT” censor never initiators 2. “As-treated” allow cross-over censor non-initiators or discontinuers 3. “Per-protocol population” censor if never initiate, cross-over, or discontinuation 4. Adherence adjustment include adherence in model for outcome model 5. Instrumental variables, aka compare outcome by trial arm, and correct 38Murray. 2019. Pragmatic randomized
- 39. Per-protocol analyses in the literature Approach Description 1. “Modified ITT” censor never initiators 2. “Per-protocol population” censor if never initiate, cross-over, or discontinuation 3. “Per-protocol population” censor if never initiate, cross-over, or discontinuation 4. Adherence adjustment include adherence in model for outcome model 5. Instrumental variables, aka compare outcome by trial arm, and correct 39Murray. 2019. Pragmatic randomized
- 40. Per-protocol analyses in the literature Approach Description 1. “Modified ITT” censor never initiators 2. “Per-protocol population” censor if never initiate, cross-over, or discontinuation 3. “As-treated” allow cross-over censor non-initiators or discontinuers 4. Adherence adjustment include adherence in model for outcome model 5. Instrumental variables, aka compare outcome by trial arm, and correct 40 Methods 1 to 3: Censor without adjustment Murray. 2019. Pragmatic randomized
- 41. Per-protocol analyses in the literature Approach Description 1. “Modified ITT” censor never initiators 2. “Per-protocol population” censor if never initiate, cross-over, or discontinuation 3. “As-treated” allow cross-over censor non-initiators or discontinuers 4. Adherence adjustment include adherence in model for outcome model 5. Instrumental variables, aka compare outcome by trial arm, and correct 41 Methods 1 to 3: Censor without adjustment Method 4: Adjustment for baseline confounding only Murray. 2019. Pragmatic randomized
- 42. Potential per-protocol analyses Approach Description 1. “Modified ITT” censor never initiators 2. “Per-protocol population” censor if never initiate, cross-over, or discontinuation 3. “As-treated” allow cross-over censor non-initiators or discontinuers 4. Adherence adjustment include adherence in model for outcome model 5. Instrumental variables, aka compare outcome by trial arm, and correct 42 Methods 1 to 3: Censor without adjustment Method 4: Adjustment for baseline confounding only Murray. 2019. Pragmatic randomized
- 43. But isn’t adherence intractably confounded? 43 Coronary Drug Project. 1980, NEJM; 303: 1038-41. Murray. 2019. Pragmatic randomized 0 5 10 15 20 25 30 Unadjusted Baseline adjusted Non-adherers Adherers 5-year mortality risk in CDP placebo arm
- 44. Effects are different from analyses Per-protocol effect tells us “how would trial outcomes differ if everyone adhered to assignment A versus if everyone adhered to assignment B” 44Murray. 2019. Pragmatic randomized
- 45. Better per-protocol analyses Approach Description 1. “Modified ITT” censor never initiators 2. “As-treated” allow cross-over censor non-initiators or discontinuers 3. “Per-protocol population” censor if never initiate, cross-over, or discontinuation 4. Adherence adjustment include adherence in model for outcome model 5. Instrumental variables, aka compare outcome by trial arm, and correct 45 Methods 1 to 3: Censor without adjustment Method 4: Adjustment for baseline confounding only Per-protocol effect estimation censor if deviate from protocol or include adherence in outcome model adjust for censoring or time-varying confounding Murray. 2019. Pragmatic randomized
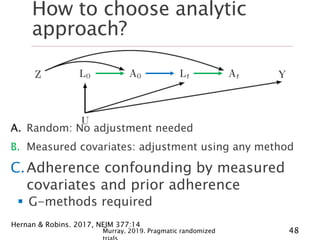
- 46. What do we need to adjust for? Hernan & Robins. 2017, NEJM 377:14 A.Random non-adherence No confounding adjustment needed 46Murray. 2019. Pragmatic randomized
- 47. How to choose analytic approach? Hernan & Robins. 2017, NEJM 377:14 A. Random: No adjustment needed B. Adherence confounding by measured covariates Adjustment required using any method 47Murray. 2019. Pragmatic randomized
- 48. How to choose analytic approach? Hernan & Robins. 2017, NEJM 377:14 A. Random: No adjustment needed B. Measured covariates: adjustment using any method C.Adherence confounding by measured covariates and prior adherence G-methods required 48Murray. 2019. Pragmatic randomized
- 49. How to choose analytic approach? Hernan & Robins. 2017, NEJM 377:14 A. Random: No adjustment needed B. Measured covariates: adjustment using any method C. Measured covariates & adherence: g-methods D.Adherence confounding by measured covariates, prior adherence, and unmeasured covariates Strong assumptions + structural nested 49Murray. 2019. Pragmatic randomized
- 50. A very quick intro to g- methods 1. Inverse probability weighting 2. G-formula 3. G-estimation 50Murray. 2019. Pragmatic randomized
- 51. G-methods generalize estimation to treatment- confounder feedback 51Murray. 2019. Pragmatic randomized
- 52. G-methods are roughly similar to … Inverse probability weighting G-formula G-estimation 52Murray. 2019. Pragmatic randomized Propensity scores Standardization Instrumental variables ≈ ≈ ≈
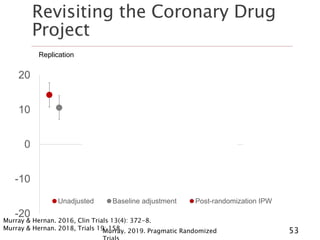
- 53. Revisiting the Coronary Drug Project Murray. 2019. Pragmatic Randomized 53 -20 -10 0 10 20 Unadjusted Baseline adjustment Post-randomization IPW Replication Cumulative incidence model Survival model, censoring Survival model, flexible dose- response Survival model, quadratic dose-response Murray & Hernan. 2016, Clin Trials 13(4): 372-8. Murray & Hernan. 2018, Trials 19: 158.
- 54. Estimating the per-protocol effect One approach: inverse probability weighting Step 1: define adherence to the protocol Step 2: build inverse probability weights for adherence and fit separately in each trial arm Step 3 [optional]: censor when non- adherent Step 4: fit an IP-weighted model for the outcome given trial arm, baseline covariates, and [optional] dose- 54Murray. 2019. Pragmatic randomized
- 55. Part V: Some guidelines for patient-centered causal effects from pragmatic trials
- 56. Start by considering the goal(s) FDA approval Clinical decision-making Patient-clinician shared decision-making Other Take home: What causal effect(s) do you need to know to achieve this goal? 56Murray. 2019. Pragmatic randomized
- 57. Choose an analytic approach that answers the causal question Randomization to treatment Initiation of treatment among ‘compliers’ Initiation of treatment among everyone Sustained treatment (protocol) Intention-to-treat & stratified ITT Per-protocol with instrumental variables Per-protocol effect estimation 57Murray. 2019. Pragmatic randomized
- 58. Designing patient-centered trials Patients mistrust non-inferiority Stratified effects are patient- centered effects Patients assess per-protocol effects based on reasons for non- adherence Patients want to Choose superiority goals Involve patients/ advocates in choosing a priori strata Include information about why participants don’t adhere Report risks &58Murray. 2019. Pragmatic randomized
- 59. Finally, don’t forget about loss to follow-up! Loss to follow-up can cause bias away from the null even in the intention-to-treat effect! Loss to follow-up is inherently post-randomization, so requires adjustment for post-randomization confounding Solutions: Inverse probability weighting G-formula Multiple imputation 59Murray. 2019. Pragmatic randomized
- 60. Draft pragmatic trial guidelines Murray. 2019. Pragmatic randomized 60 Choice of causal effect 1. Report estimates of both the intention-to-treat effect and the per-protocol effect. 2. Report absolute risks and their differences, as well as their ratios, for discrete outcomes. 3. Heterogeneity of treatment effects can be reported using subgroup analyses that use the additive scale. 4. Pre-specify important prognostic factors & maximum acceptable difference between groups; adjust via standardization or inverse probability weighting. 5. In sensitivity analyses, adjust for large imbalances in any important prognostic factors, regardless of pre- specification. 6. In survival analyses with competing events, specify the intention-to-treat effect as the total effect of treatment assignment; conduct sensitivity analyses
- 61. Draft pragmatic trial guidelines Murray. 2019. Pragmatic randomized 61 Valid effect estimates in the presence of loss to follow-up 7. Collect post-randomization time-varying prognostic factors that predict loss to follow-up, & use g- methods to adjust. Estimating the per-protocol effect of a point intervention 8. Per-protocol effects of point interventions can be estimated using inverse probability weighting or standardization. 9. Estimate bounds for the per-protocol effect of point interventions when the instrumental conditions are expected to hold for treatment assignment. Provide a justification for the exclusion restriction. 10. When instrumental conditions and monotonicity hold, discuss whether the effect in the “compliers” is of interest. If so, estimate it & provide information on the relative size and characteristics of the “compliers”
- 62. Draft pragmatic trial guidelines Murray. 2019. Pragmatic randomized 62 Estimating the per-protocol effect for a sustained intervention 11. Specify a priori a treatment protocol that incorporates real world clinical decision-making. When there is sufficient ambiguity about appropriate strategies, more than 1 can be specified. 12. Collect sufficient data to determine adherence throughout the follow-up, and to adjust for time- varying prognostic factors that predict adherence. 13. Use g-methods to adjust for time-varying confounders when there is treatment-confounder feedback.
- 63. Where to get more information Some references: Proposed guidelines: https://www.hsph.harvard.edu/causal/pragmatictrials/ Patient-centered causal effects: Murray et al. 2018. J Clin Epi 103:10-21. Choosing a causal effect: Hernan & Scharfstein. 2018, Ann Intern Med; 168(7):515-6. Per-protocol effect estimation: Hernan & Robins. 2017, NEJM 377:14; Lodi et al, 2016. AIDS; 30(17):2659-63. Placebo arm adherence analyses: Murray & Hernan. 2018, Trials 19:158; Murray & Hernan. 2016, Clin Trials 13(4): 372-8. G-methods: Causal Inference, Hernan & Robins. Available online at: https://www.hsph.harvard.edu/miguel-hernan/causal-inference-book/ Contact me: @EpiEllie ejmurray@bu.edu https://github.com/eleanormurray 63Murray. 2019. Pragmatic randomized
Editor's Notes
- #34: Note with these definitions, we don’t need to distinguish between per-protocol versus as-treated







![Pragmatic randomized
trials
https://www.gopubmed.org/web/gopubmed/statistics/”Pragmatic+Clinical+Trials
+as+Topic”[mesh]
7Murray. 2019. Pragmatic randomized](https://image.slidesharecdn.com/umichigan-190514022613/85/Causal-Inference-from-Pragmatic-Trials-7-320.jpg)







![Patients who expect to adhere may
change their choices based on per-
protocol effects
“It would depend on how critical
the case was. If I had serious
COPD and there was, both
parents had died of it, I would
say, ‘You know what? I am
committed to my health. I’m
committed to taking it as
prescribed.’ So I’d be willing to
try the new [less convenient]
drug.”
15Murray. 2019. Pragmatic randomized
Murray et al. 2018 J Clin
Trials, 103:10-21.](https://image.slidesharecdn.com/umichigan-190514022613/85/Causal-Inference-from-Pragmatic-Trials-15-320.jpg)




































![Estimating the per-protocol
effect
One approach: inverse probability
weighting
Step 1: define adherence to the protocol
Step 2: build inverse probability weights
for adherence and fit separately in each
trial arm
Step 3 [optional]: censor when non-
adherent
Step 4: fit an IP-weighted model for the
outcome given trial arm, baseline
covariates, and [optional] dose- 54Murray. 2019. Pragmatic randomized](https://image.slidesharecdn.com/umichigan-190514022613/85/Causal-Inference-from-Pragmatic-Trials-54-320.jpg)








