Cdsco ppt
- 1. Central Drug Standard Control Organisation (CDSCO) 1 Presented by: Nikhat Parveen
- 2. OVERVIEW Introduction: CDSCO Functions of CDSCO Organisational Chart Clinical Trial process Drug approval process Cosmetics & Medical devices approval SUGAM: Online portal Recent advances in CDSCO Summary References 2
- 3. INTRODUCTION: The Central Drugs Standard Control Organization (CDSCO) is the national regulatory body for Indian pharmaceuticals and medical devices. It serves parallel function to the European Medicines Agency of the European Union, the PMDA of Japan, the Food and Drug Administration of the United States and the Medicines and Healthcare products Regulatory Agency of the United Kingdom. Headquarters: New Delhi, India Ministry responsible: Ministry of Health and Family Welfare. Minister responsible: Harsh Vardhan.(1) 3
- 4. DRUG CONTROLLER GENERAL OF INDIA Within the CDSCO, the Drug Controller General Of India (DCGI), regulates pharmaceutical and medical devices, under the gamut of Ministry of Health and Family Welfare. He/She is responsible for approval of new drugs, medical devices, and Clinical Trial to be conducted in India The DCGI is advised by the Drug Technical Advisory Board (DTAB) and the Drug Consultative Committee (DCC). DCGI: Dr. S. Eswara Reddy(1) 4
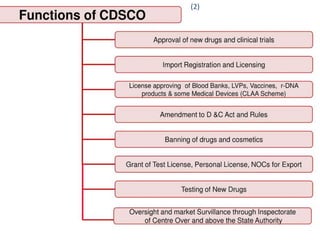
- 5. 5 (2)
- 6. ORGANISATIONAL CHART OF CDSCO 6 (3)
- 7. CLINICAL TRIAL PROCESS An application to conduct clinical trials in India should be submitted along with the data of chemistry, manufacturing, control and animal studies to DCGI. The date regarding the trial protocol, investigator's brochures, and informed consent documents should also be attached. A copy of the application must be submitted to the ethical committee and the clinical trials are conducted only after approval of DCGI and ethical committee. To determine the maximum tolerated dose in humans, adverse reactions, etc. on healthy human volunteers, Phase I clinical trials are conducted. The therapeutic uses and effective dose ranges are determined in Phase II trials in 10-12 patients at each dose level. The confirmatory trials (Phase III) are conducted to generate data regarding the efficacy and safety of the drug in ~ 100 patients (in 3-4 centers) to confirm efficacy and safety claims. Phase III trials should be conducted on a minimum of 500 patients spread across 10-15 centers. (4) 7
- 8. If the new drug substance is not marketed in any other country. The new drug registration (using form 44 along with full pre- clinical and clinical testing information) is applied after the completion of clinical trials. The comprehensive information on the marketing status of the drug in other countries is also required other than the information on safety and efficacy. The information regarding the prescription, samples and testing protocols, product monograph, labels, and cartons must also be submitted. The application can be reviewed in a range of about 12-18 months. After the NDA approval, when a company is allowed to distribute and market the product, it is considered to be in Phase IV trials, in which new uses or new populations, long- term effects, etc. are explored. (4) 8
- 10. When a company in India wants to manufacture/ import a new drug it has to apply to seek permission from the licensing authority (DCGI) by filing in Form 44 also submitting the data as given in schedule Y of D & C act 1940 and rules 1945. Applicants are required to provide technical data in respect of safety and efficacy before these could be permitted to be marketed in country. Definitions of new drug also includes fixed dose combination which are required to be marketed for the 1st time in country. (4) 10
- 11. COSMETICS & MEDICAL DEVICES APPROVAL For the import of cosmetics into India, the cosmetic products need to be registered with the licensing authority as defined under Rule 21 of Drugs & Cosmetic Rules. The regulatory application needs to be submitted in Form 42 along with Soft copies of the information about the brands, products and manufacturer, product specification and testing protocol to receive the cosmetics registration certificate in Form 43. The Cosmetics that are supposed to be imported are categorized into Brands. These brands are divided into 4 main category namely Skin products, Hair and scalp products, nail and cuticle products and Oral hygiene products. License will be granted within 6 months by CDSCO. However, as per a notification in 2014, the targeted timeline for cosmetics regulatory approval process is 90 days. (5) 11
- 12. Earlier, manufacturers could sell medical devices in India without any jurisdictions. Since 2006, medical devices entering India must be in compliance with the Indian Medical Device Regulation has been set by the CDSCO. Registration Certificate in Form-41 and Import License in Form-10 are required under the regulation of Drugs and Cosmetics Act, 1940, for marketing of imported medical devices. Medical devices which undergo regulatory approval process include spinal needles, cochlear implants, annuloplasty rings, tracheostomy tubes, syringes and needle, dental implants, surgical sealants, heart valves, cardiac stents, orthopedic implants, endotracheal tubes, and catheters. The time period for Registration is generally 6-9 months, post the submission of complete and accurate regulatory dossier and fees. Registration is valid for 3 years and renewal applications (re- registration) need to be submitted 6 months in advance of expiry of the registration certificate. (5) 12
- 13. RECENT ADVANCES IN CDSCO To improve ease of doing business, CDSCO increases the validity of the WHO GMP certificate to three years. Earlier it was valid for two years, said DCGI, Eswara Reddy during the 6th annual International Exhibition of Pharma and Healthcare. The protocol for clinical trial approvals has also been changed by setting the timeline of approval at 45 days. Once approval does not come within this timeline, the protocol would deemed to be approved. 56th DCC held on 01st June 2019, DCC after deliberation opined that New Drugs and Clinical Trials Rules, 2019 for mandating inspections of BA/BE study centres by officers authorized by State Licensing Authorities. 82nd DTAB held on 02.04.2019, examine the Safety and Efficacy of unapproved FDCs which were licensed by State Licensing Authorities without due approval of DCG(I). (6) 13
- 14. After examining 418 applications of FDCs, the Kokate Committee has found 324 FDCs as irrational, after evaluating all the data submitted and available information, 28 FDCs as rational, 2 FDCs which require further generation of data and 4 FDCs which require further deliberation. It was also observed that 60 FDCs have already been either prohibited (48 FDCs) or have been already declared as rational (11 FDCs), or fall under sub-judice category (1 FDC) by the Kokate Committee which were placed in the list of these 418 applications of FDCs inadvertently. Therefore, the Kokate Committee did not make any recommendations on these 60 applications of FDCs. Minutes of IND Committee meeting held on 15.03.2019 and again held on 10.06.2019 on the Clinical trial with Rabimab of M/s Cadila Healthcare Limited. (6) 14
- 15. SUGAM: ONLINE PORTAL An online licensing portal of CDSCO has been implemented on January 2016 and has been named “SUGAM” to file applications for various services like Application Submission, Processing and Grant of permission for quick delivery of services. SUGAM BENEFITS: Applicant can apply for license under import & registration division to CDSCO. Track the status of submitted application. Answer back to the raised queries. Applicant can also upload essential documents for Registration, import license & other related activities. (7) 15
- 16. SUMMARY CDSCO is main regulatory body for Indian pharmaceuticals, medical devices and clinical trial. Head office is located in New Delhi, and functioning under the control of DCGI, under ministry of MoHFW. There are 6 zonal offices, 3 sub-zonal and 6 laboratories are under CDSCO, to perform the GMP audits & inspection in manufacturing units, uniform standard of inspection and enforcement and quality control of drugs & cosmetics in the country respectively. Clinical trial approval, drug approval, cosmetics and medical devices approval all are done with the permission granted by DCGI after ensuring that all submitted documents are accurate and complete. 16
- 17. REFERENCES 1. https://en.wikipedia.org/wiki/Central_Drugs_Standard_Contr ol_Organization 2. https://cdsco.gov.in/opencms/opencms/en/About- us/Functions/ 3. https://cdsco.gov.in/opencms/export/system/modules/CDSC O.WEB/resources/pdf/Introduction/org.pdf 4. https://sites.google.com/site/athonycrastopharmaceuticals/dru g-a 5. https://morulaa.com/registration-services-in-india-cdsco/ 6. https://cdsco.gov.in/opencms/opencms/en/Committees/ 7. https://morulaa.com/cdsco/sugam-online-portal-a-brief- overview/ 17
- 18. 18