Fourier-Transform Infrared (FTIR) and Raman Spectroscopy
- 1. FOURIER-TRANSFORM INFRARED (FTIR) AND RAMAN SPECTROSCOPY An Overview of Techniques and Applications Presented by: Natheir Hasan Date: 6th November 2024
- 3. WHAT IS SPECTROSCOPY? Spectroscopy is the scientific study of the interaction between electromagnetic radiation (light) and matter. It encompasses various techniques used to analyze the composition and structure of substances by examining the light they absorb, emit, or scatter.
- 4. LIGHT-MATTER INTERACTION Light and matter interact with each other in various ways. Matter can absorb light (take it in); emit light (give it off); transmit light (allow it to pass through); reflect light (cause it to bounce off); and refract light (cause it to change speed and direction).
- 5. IMPORTANCE OF SPECTROSCOPY IN CHEMICAL ANALYSIS: • It does not alter or damage the sample being analyzed. Non-Destructive Analysis: • Provides detailed information about the molecular structure and composition of substances. Detailed Molecular Information: • Pharmaceuticals, Environmental Science, Materials Science Wide Range of Applications:
- 6. TYPES OF SPECTROSCOPY Emission spectroscopy • Fluorimetry • Flame photometry • Atomic Emission Spectroscopy Absorption Spectroscopy • UV-Visible Spectroscopy • IR Spectroscopy • Nuclear Magnetic Resonance Spectroscopy • Atomic Absorption Spectroscopy Scattering Spectroscopy • Raman Spectroscopy
- 9. DEFINITION OF IR SPECTROSCOPY IR Spectroscopy (Infrared Spectroscopy) is an analytical technique used to identify and study chemicals by measuring how molecules absorb infrared light. This absorption causes molecular vibrations, such as stretching and bending of bonds, which are unique to different functional groups.
- 10. PRINCIPLE • When a molecule absorbs IR radiation, the energy of the radiation matches the energy difference between the molecule's vibrational states. • Only vibrational transitions that lead to a change in the dipole moment of the molecule will absorb IR light (making polar bonds important). • The absorption of IR radiation occurs at specific wavelengths (wavenumbers), corresponding to the natural vibrational frequencies of the bonds in the molecule.
- 11. PRINCIPLE • Nonpolar molecules may not show significant IR absorption unless a dipole moment is induced during vibration. • Mid-IR region ( cm) is where most fundamental vibrations occur and is the most commonly used region in IR spectroscopy.
- 12. IR REGION IN THE ELECTROMAGNETIC SPECTRUM
- 13. VIBRATIONAL MODES Radial (Stretching) Symmetric stretching Antisymmetric stretching Latitudinal (In plane bending) Scissoring Rocking Longitudinal (Out of plane bending) Wagging Twisting
- 14. VIBRATIONAL MODES • For non-linear molecules with N atoms, the number of vibrational modes is given by: The is the total degrees of freedom of the molecule and the translational 3 degrees of freedom, and 3 rotational are then subtracted which leaves 3N – 6 vibrational modes. • For linear molecules, there is one less rotational degree of freedom and the number of vibrational modes is therefore:
- 15. QUANTUM MECHANICAL BASIS • Each vibrational mode corresponds to a specific vibrational energy level in the molecule. • These energy levels are quantized, meaning the molecule can only vibrate at specific frequencies. • Only vibrations that cause a change in the dipole moment of the molecule are IR- active. Therefore, not all vibrations will result in IR absorption.
- 16. DISPERSIVE INFRARED SPECTROSCOPY Traditional IR spectroscopy begins by shining IR light onto a diffraction grating, which separates light by wavelength. Different wavelengths exit the grating at slightly different angles due to the relationship between the angle of exit and the wavelength of light. This technique is called dispersive infrared spectroscopy because it disperses the wavelengths in space.
- 17. DISPERSIVE INFRARED SPECTROSCOPY After the IR beam is separated by wavelength, it passes through a slit to create a monochromatic beam directed at the sample for absorbance measurement. The diffraction grating angle is then adjusted to isolate other wavelengths, repeating the process until all absorbances are collected, which allows for the plotting of the IR spectrum.
- 18. DISPERSIVE INFRARED SPECTROSCOPY The issue with this dispersive design was the long time it took to obtain an entire spectrum due to the sequential detection.
- 19. IR SPECTRUM
- 20. THE FINGERPRINT REGION • Complex Absorptions: The region below 1500 cm is known as the fingerprint region. It contains a dense series of absorption peaks that are unique to the molecule. • This region arises from bending vibrations, complex stretching modes, and out-of-plane vibrations. • Identification: The fingerprint region is highly specific to each molecule and can be used to match a sample against known reference spectra for compound identification. • Bending vibrations dominate.
- 21. THE DIAGNOSTIC REGION • The diagnostic region in IR spectroscopy spans from 4000 cm to 1500 cm. • It is called the diagnostic region because it contains well-defined absorption peaks that are easily correlated with specific functional groups. • In contrast to the fingerprint region, peaks in this region are less crowded and easier to interpret, making it ideal for identifying functional groups. • Stretching vibrations dominate.
- 22. KEY FUNCTIONAL GROUPS AND THEIR IR ABSORPTION RANGES • Each functional group in a molecule absorbs IR radiation at characteristic wavenumbers due to its unique bond vibrations. • The position and intensity of these absorption bands allow for the identification of specific groups in a molecule.
- 24. DEFINITION • Fourier Transform Infrared Spectroscopy (FTIR) is an advanced analytical technique that measures the infrared absorption of a sample, providing insights into its molecular composition and structure. • Unlike traditional infrared spectroscopy, which typically employs monochromatic light sources, FTIR utilizes a broadband infrared source. This means that FTIR simultaneously collects data across a wide range of wavelengths (or frequencies) instead of measuring one wavelength at a time.
- 26. FOURIER-TRANSFORM IR SPECTROMETER 1. IR Source: Emits a broad spectrum of infrared radiation (4000 cm 400 cm) that interacts with the sample. Types of Sources: 2. Globar 3. Tungsten-Halogen lamp
- 27. FOURIER-TRANSFORM IR SPECTROMETER 2. Beam-Splitter: Divides the incoming infrared beam into two paths: one directed toward a fixed mirror and the other toward a moving mirror. It is typically made from materials like or germanium.
- 28. FOURIER-TRANSFORM IR SPECTROMETER 3. Fixed Mirror: Reflects one of the beams back toward the beam splitter. 4. Moving Mirror: Adjusts the path length of the second beam, causing changes in phase that create interference patterns.
- 29. FOURIER-TRANSFORM IR SPECTROMETER • Constructive interference occurs when the peaks of the waves align, reinforcing each other and increasing the signal intensity at certain wavelengths.
- 30. FOURIER-TRANSFORM IR SPECTROMETER • Destructive interference happens when the peak of one wave aligns with the trough of another, canceling each other out, reducing the signal at those wavelengths.
- 31. FOURIER-TRANSFORM IR SPECTROMETER • This pattern of alternating constructive and destructive interference creates a signal called an interferogram. • The interferogram contains information about all wavelengths of light that passed through the sample and is later converted into a spectrum using Fourier Transform.
- 32. FOURIER-TRANSFORM IR SPECTROMETER 5. Sample Holders • Solid Samples: Can be analyzed directly or prepared as thin films, often using techniques like KBr pellets. • Liquid Cells: Typically made of materials like KBr. • Gas Cells: Designed with long path lengths and reflective mirrors, used for gaseous samples.
- 33. FOURIER-TRANSFORM IR SPECTROMETER 6. Detector: Converts the infrared light that has passed through or reflected from the sample into an electrical signal for analysis. Mercury Cadmium Telluride (MCT): A semiconductor detector that is sensitive to a wide range of IR wavelengths.
- 34. FOURIER-TRANSFORM IR SPECTROMETER 7. Inteferogram: A graph showing the raw signal from the detector.
- 35. FOURIER-TRANSFORM IR SPECTROMETER 7. Inteferogram: An interferogram is a graph showing the raw signal from the FTIR detector. The x-axis represents the path difference (retardation) between light beams, and the y-axis shows the intensity of transmitted light. It displays intensity variations due to light interference and contains the data needed for spectrum analysis.
- 36. FOURIER-TRANSFORM IR SPECTROMETER 8. Computer/FT Algorithm: The Fourier Transform (FT) algorithm converts the interferogram from the time domain into a spectrum, enabling the analysis of molecular vibrations by displaying the intensity of transmitted light as a function of wavenumber (or frequency).
- 37. IR SPECTRUM
- 38. IR SPECTRUM
- 39. IR SPECTRUM An even more compressed overview looks like this: Wavenumuber ) Bond Type 3600 – 2700 (single bonds to hydrogen) 2700 – 1900 (triple bonds) 1900 – 1500 (double bonds) 1500 – 500 (Single bonds)
- 40. D-GLUCOSE IR SPECTRUM The figure to the left shows the IR spectrum of D- glucose. The spectrum shows the transmittance as a function of wavenumber.
- 41. D-GLUCOSE IR SPECTRUM • present around cm. • No stretch present. No strong peak around 1700 cm . • No alkene (no peaks above 3000 cm )
- 42. USING DATABASES TO IDENTIFY BONDS What functional groups (types of bonds) are present in the IR spectrum shown?
- 43. USING DATABASES TO IDENTIFY BONDS In the analytical region, the spectrum has a broad-tongue like peak around 3300 cm, and two medium-sharped peaks between 2950 and 2800 cm.
- 44. USING DATABASES TO IDENTIFY BONDS Using the Database: • The broad tongue- like peak around 3300 cm is typical of • All stretches are below 3000 cm
- 45. USING DATABASES TO IDENTIFY BONDS We also notice that: • There is no strong peak around 1700 cm, which indicates that there are no bonds.
- 46. IR SPECTRUM OF POLYPROPYLENE • The Peak observed at 2952 cm is related to asymmetric stretching vibration. • The peaks at 1455, 2838 and 2917 cmare attributed to symmetric bending, symmetric stretching and asymmetric stretching.
- 47. IR SPECTRUM OF POLYPROPYLENE • Symmetric bending vibration mode of group is detected at 1375 cm. • The peaks displayed at 972, 997 and 1165 cm are assigned to rocking vibration. • The peak located at 840 cm is assigned to stretching vibration.
- 48. IR SPECTRUM OF CYCLIC OLEFIN COPOLYMER • The absorption bands sited at 2866 and 2941 cm are assigned to the hybridized carbon atoms symmetric and asymmetric stretching vibrations. • IR peak exhibited at 1452 cm corresponds to bending vibration mode of group.
- 49. IR SPECTRUM OF CYCLIC OLEFIN COPOLYMER • IR peaks displayed at 934 and 1254 cm region are attributed to bending vibration. • Absorption peak displayed at 1294 cm region is related to stretching vibration mode of backbone.
- 50. ADVANTAGES AND DISADVANTAGES Advantages • High Sensitivity • Rapid Data Collection • Broad Range of Applications disadvantages • Water Interference • Limited to Certain Molecules • Complexity in Mixtures
- 52. DEFINITION OF SCATTERING SPECTROSCOPY Scattering spectroscopy is a technique that examines how light interacts with matter by analyzing the scattering of light from a sample. It focuses on the changes in wavelength and intensity of the scattered light to provide information about the molecular structure and composition of the sample, distinguishing between elastic (Rayleigh) and inelastic (Raman) scattering.
- 53. TYPES OF SCATTERING 1. Elastic Scattering (Rayleigh Scattering): • Scattering of light by particles without a change in wavelength or energy. • Occurs when photons interact with small particles, retaining the same energy as the incident light.
- 54. TYPES OF SCATTERING • Intensity decreases with increasing wavelength (shorter wavelengths scatter more). • Explains why the sky appears blue.
- 55. TYPES OF SCATTERING 2. Inelastic Scattering (Raman Scattering): • Scattering of light produces scattered photons with a different frequency depending on the source and the vibrational and rotational properties of the scattered molecules
- 56. TYPES OF SCATTERING 2. Inelastic Scattering (Raman Scattering): Photons are either: • Stokes Scattering: Lose energy to the molecule, resulting in longer wavelengths. • Anti-Stokes Scattering: Gain energy from the molecule, resulting in shorter wavelengths.
- 57. TYPES OF SCATTERING 2. Inelastic Scattering (Raman Scattering): • Provides specific information about molecular vibrations and chemical bonds. • The intensity of Raman signals depends on the molecular concentration and structure.
- 58. RAMAN SCATTERING When light is scattered by a molecule, the photon's electromagnetic field polarizes the molecule’s electron cloud, briefly raising the molecule to a higher energy state. This forms a short-lived where the energy is temporarily absorbed. The molecule quickly returns to a stable state, re-emitting the photon as scattered light.
- 59. RAMAN SCATTERING In rare cases (about 1 in a million photons), Raman scattering occurs, an inelastic process where energy is transferred between the molecule and the photon.
- 60. RAMAN SCATTERING If the molecule gains energy, the scattered photon loses energy, increasing its wavelength—this is known as Stokes Raman scattering.
- 61. RAMAN SCATTERING If a molecule loses energy by relaxing to a lower vibrational level, the scattered photon gains energy, decreasing its wavelength—this is called Anti-Stokes Raman scattering.
- 62. RAMAN SCATTERING • Rayleigh Scattering: The photon’s energy remains unchanged as it is scattered elastically. • Stokes Raman Scattering: The molecule gains energy, while the scattered photon loses energy. • Anti-Stokes Raman Scattering: The molecule loses energy, and the scattered photon gains energy.
- 63. RAMAN SCATTERING • Rayleigh Scattering (middle peak): Shows no shift in frequency. • Stokes Raman Shift (right peak): The scattered photon has a higher wavelength. • Anti-Stokes Raman Shift (left peak): The scattered photon has a lower wavelength.
- 65. DISPERSIVE RAMAN SPECTROMETERS Dispersive Raman spectroscopy is one of the most common types of Raman spectroscopy. It works by dispersing the scattered light from a sample into its component wavelengths, providing detailed information about molecular vibrations, crystal structures, and material composition.
- 66. DISPERSIVE RAMAN SPECTROMETERS 1. Laser Source: A monochromatic laser source, typically in the visible or near-infrared range, irradiates the sample. Common lasers include Argon-ion (514.5 nm) and Nd (1064 nm) lasers.
- 67. DISPERSIVE RAMAN SPECTROMETERS 2. Sample Holder: • Solid Holders: For bulk materials, thin films, and powders. • Liquid Cells: Quartz or glass cuvettes for liquids. • Gas Cells: Airtight containers for gases. • Microscope Holders: For precise focusing on small areas.
- 68. DISPERSIVE RAMAN SPECTROMETERS 3. Notch Filter • Blocks the intense Rayleigh scattering that occurs when the laser interacts with the sample. It allows only the Raman-shifted light to pass through to the detector.
- 69. DISPERSIVE RAMAN SPECTROMETERS 4. Diffraction Grating: The diffraction grating separates scattered light into different wavelengths to enable the detection of Raman shifts, allowing for the analysis of molecular vibrations and composition in the sample.
- 70. DISPERSIVE RAMAN SPECTROMETERS 5. CCD detector • Detects the dispersed Raman signals after they pass through the diffraction grating, capturing the intensity and wavelength of the light. • Charge-Coupled Device detectors are highly sensitive to low light levels
- 71. DISPERSIVE RAMAN SPECTROMETERS The final step produces a Raman spectrum, with intensity on the vertical axis and wavenumber on the horizontal. Peaks in the spectrum represent molecular vibrations, revealing the sample's chemical structure and composition.
- 72. FOURIER-TRANSFORM RAMAN SPECTROMETERS FT-Raman spectroscopy employs a laser source to excite a sample, generating scattered light that carries information about molecular vibrations. An interferometer modulates this light, producing an interference pattern that is detected and processed by a computer to convert the time- domain signal into a frequency- domain spectrum.
- 73. FOURIER-TRANSFORM RAMAN SPECTROMETERS The interferometer Modulates the scattered light by splitting it into two beams using a beamsplitter. One beam reflects off a fixed mirror, while the other reflects off a movable mirror. When the beams recombine, they create an interference pattern that encodes spectral information.
- 74. FOURIER-TRANSFORM RAMAN SPECTROMETERS The pattern of alternating constructive and destructive interference generates a signal known as an interferogram. This interferogram contains information about all wavelengths of the Raman-scattered light from the sample and is subsequently converted into a spectrum using Fourier Transform.
- 75. FOURIER-TRANSFORM RAMAN SPECTROMETERS The Fourier Transform (FT) algorithm converts the interferogram from the time domain into a spectrum, enabling the analysis of molecular vibrations by displaying the intensity of scattered light intensity as a function of wavenumber (or Raman Shift).
- 76. RAMAN SPECTRUM • Each peak on the Raman spectrum corresponds with a different frequency of light absorbed by the sample which excited a vibration. • Since these frequencies are unique to the molecule and the types of bonds it contains, the Raman spectra creates a “chemical fingerprint” that allows us to identify and quantify a large variety of substances.
- 77. RAMAN SPECTRUM For example, we know oxygen vibrates at around 1550 cm while nitrogen vibrates at 2330 cm. If we illuminate these gases with our green laser, the gases will absorb those frequencies of light which we’ll see on the Raman spectra. There will be a peak for oxygen around 1550 cm and a peak for nitrogen around 2330 cm.
- 78. RAMAN SPECTRUM OF CARBON TETRACHLORIDE (CCL4) • In the center of the spectrum is the Rayleigh scatter peak at the laser wavelength. This peak is millions of times more intense than the Raman scatter and is therefore normally blocked by a notch filter in the Raman spectrometer but was included here for clarity.
- 79. RAMAN SPECTRUM OF CARBON TETRACHLORIDE (CCL4) Symmetrically placed on either side of the Rayleigh peak are the three Stokes and three Anti-Stokes peaks corresponding to the three most intense Raman active vibrations of CCl4.
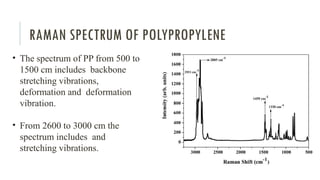
- 80. RAMAN SPECTRUM OF POLYPROPYLENE • The spectrum of PP from 500 to 1500 cm includes backbone stretching vibrations, deformation and deformation vibration. • From 2600 to 3000 cm the spectrum includes and stretching vibrations.
- 81. RAMAN SPECTRUM OF CYCLIC OLEFIN COPOLYMER • The band at 932 cm represents the ring vibrations of COC molecules. • The sharp peak at 1416 cm is characterized for an orthorhombic crystal structure in polyethylene • Raman peaks at 1225 and 1311 cm are related to the twisting mode of the group in COC.
- 82. RAMAN SPECTRUM OF CYCLIC OLEFIN COPOLYMER • The observed IR band at 1041 and 1118 cm corresponds to the stretching vibrations of backbone. • The absorption bands displayed at 2870 and 2924 cm are assigned to symmetric and asymmetric stretching vibrations, respectively.
- 83. ADVANTAGES AND DISADVANTAGES Advantages • Minimal water interference • Can analyze a wide range of material • Minimal to no sample preparation disadvantages • Weak signal • Fluorescence interference • Some samples may be damaged by the laser • Sensitivity to noise
- 84. COMPARISON BETWEEN FTIR AND RAMAN SPECTROSCOPY Fourier Transform Infrared Spectroscopy Raman Spectroscopy Principle Measures the absorption of infrared light, causing molecular vibrations. Measures the scattering of light caused by changes in polarizability during molecular vibrations. Sample Preparation Requires more preparation, especially for solid samples (e.g., pressing into pellets). Minimal to no sample preparation; can analyze directly. Water Interference Strong interference from water absorption Minimal interference from water
- 85. COMPARISON BETWEEN FTIR AND RAMAN SPECTROSCOPY Fourier Transform Infrared Spectroscopy Raman Spectroscopy Best For Polar bonds and functional groups (e.g). Best For: Non-polar bonds (e.g., ). Laser Damage No laser damage, uses infrared light. Laser Damage: Possible laser- induced damage to sensitive samples. Cost Generally more affordable More expensive due to specialized lasers and detectors.
- 86. SUMMARY: FTIR Spectroscopy is better for identifying polar bonds and is less affected by fluorescence. Raman Spectroscopy is ideal for non-polar bonds and works well with water- containing samples but is prone to fluorescence interference.
- 87. QUESTIONS?
- 88. THANK YOU!