Lecture: Microstructures in polymers
- 1. Textbook: Plastics: Materials and Processing (Third Edition), by A. Brent Young (Pearson, NJ, 2006). Structure and Properties of Engineering Polymers Lecture: Microstructures in Polymers Nikolai V. Priezjev
- 2. Microstructures in Polymers • Gas, liquid, and solid phases, crystalline vs. amorphous structure, viscosity • Thermal expansion and heat distortion temperature • Glass transition temperature, melting temperature, crystallization • Polymer degradation, aging phenomena • Molecular weight distribution, polydispersity index, degree of polymerization • Effects of molecular weight, dispersity, branching on mechanical properties • Melt index, shape (steric) effects Reading: Chapter 3 of Plastics: Materials and Processing by A. Brent Strong https://www.slideshare.net/NikolaiPriezjev
- 3. Gas, Liquid and Solid Phases Increasingdensity At room temperature Solid or liquid?
- 4. Pitch Drop Experiment http://smp.uq.edu.au/content/pitch-drop-experiment Pitch, before and after being hit with a hammer. In 1927, Professor Parnell at UQ heated a sample of pitch and poured it into a glass funnel with a sealed stem. Three years were allowed for the pitch to settle, and in 1930 the sealed stem was cut. From that date on the pitch has slowly dripped out of the funnel, with seven drops falling between 1930 and 1988, at an average of one drop every eight years. However, the eight drop in 2000 and the ninth drop in 2014 both took about 13 years to fall. It turns out to be about 100 billion times more viscous than water! Pitch (derivative of tar) at room T feels like solid and can be shattered by a hammer. But, the longest experiment shows that it flows!
- 5. Liquid phases: polymer melt vs. polymer solution Semi-dilute solution:Dilute solution: Polymer melt: Chains in solvent do not overlap: -- Can consider that each polymer chain acts in isolation. In semi-dilute solutions concentration of polymer chains is sufficient for chains to just overlap. Entangled/unentangled No solvent/only polymer chains Reptation—motion of long linear, entangled macromolecules amorphous polymers. High viscosity Low viscosity T up, viscosity down
- 6. Liquid Crystal Phases Isotropic phaseNematic phaseSmectic A Liquid crystal displays: OFF state ON state No positional but orientational order! director Stiff backbone https://www.slideshare.net/NikolaiPriezjev
- 7. Liquid Crystal Polymers Characteristics: • These are a class of aromatic polymer. • Extremely unreactive and inert. • Highly resistant to fire. Discotic nematic liquid crystals Kevlar, the most widely used body armor is made up of intertwined liquid crystal polymers.
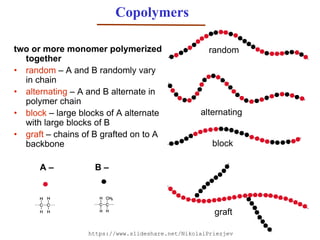
- 8. Copolymers two or more monomer polymerized together • random – A and B randomly vary in chain • alternating – A and B alternate in polymer chain • block – large blocks of A alternate with large blocks of B • graft – chains of B grafted on to A backbone A – B – random block graft alternating https://www.slideshare.net/NikolaiPriezjev
- 9. Block Copolymer Microstructures a Lamella structure. b Double gyroid (bicontinuous) structure. c Cylindrical dispersion structure. d Spherical dispersion structure.
- 10. (Di) Block Copolymer Microstructures Block copolymer microstructures and the phase diagram for the polystyrene- polyisoprene diblock copolymer system. The metastable perforated lamellar microstructure is illustrated in the upper right corner of the phase diagram. x, the relative volume fractions of the dissimilar segments N, molecular weight Structure and order in soft matter: symmetry transcending length scale. Ward and Horner CrystEngComm, 2004, 6, 401-407
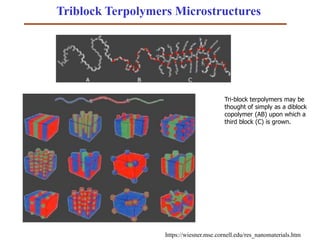
- 11. Triblock Terpolymers Microstructures https://wiesner.mse.cornell.edu/res_nanomaterials.htm Tri-block terpolymers may be thought of simply as a diblock copolymer (AB) upon which a third block (C) is grown.
- 12. Polymer Crystallinity Crystallization in linear polymers: achieving a very regular arrangement of the mers Induction of crystallinity ● cooling of molten polymer ● evaporation of polymer solution ● annealing heating of polymer at a specific temperature ● drawing stretching at a temperature above Tg Increased Density Increases Stiffness (modulus) Reduces permeability Increases chemical resistance Reduces toughness Effects of crystallinity: Semi-crystalline Amorphous https://www.slideshare.net/NikolaiPriezjev
- 13. Polymer Crystallinity Crystalline morphologies • Spherulite aggregates of small fibrils in a radial pattern (crystallization under no stress) • Drawn fibrillar obtained by drawing the spherulitic fibrils • Epitaxial one crystallite grown on another; lamella growth on long fibrils; the so-called shish-kebab morphology (crystallization under stirring) Crystalline polymers (vs amorphous polymers) tougher, stiffer (due to stronger interactions) higher density, higher solvent resistance (due to closely packing morphology) more opaque (due to light scattering by crystallites)
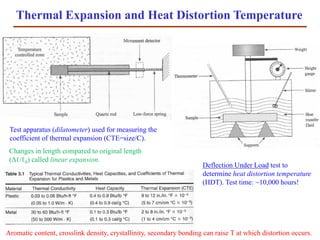
- 14. Thermal Expansion and Heat Distortion Temperature Aromatic content, crosslink density, crystallinity, secondary bonding can raise T at which distortion occurs. Test apparatus (dilatometer) used for measuring the coefficient of thermal expansion (CTE=size/C). Deflection Under Load test to determine heat distortion temperature (HDT). Test time: ~10,000 hours! Changes in length compared to original length (Δℓ/ℓ0) called linear expansion.
- 15. The Glass Transition Temperature, Tg • The glass transition, Tg, is temp. below which a polymer OR glass is brittle or glass-like; above that temperature the material is more plastic. • The Tg to a first approximation is a measure of the strength of the secondary bonds between chains in a polymer; the stronger the secondary bonds; the higher the glass transition temperature. Polyethylene Tg = 0°C; Polystyrene = 97 °C PMMA (plexiglass) = 105 °C. Since room temp. is < Tg for PMMA, it is brittle at room temp. For rubber bands: Tg = - 73°C…. https://www.slideshare.net/NikolaiPriezjev
- 16. First-order and second-order phase transitions (I) The classification of phase transitions proposed by Ehrenfest is based on the behavior of G near the phase transformation. First-order phase transition: first derivatives of G are discontinuous. Second-order phase transition: first derivatives of G are continuous, but second derivatives of G are discontinuous. G = H - TS
- 17. First-order and second-order phase transitions (II) First-order phase transition: first derivatives of G are discontinuous. Second-order phase transition: first derivatives of G are continuous, but second derivatives of G are discontinuous. G = H - TS
- 18. The Glass Transition Temperature, Tg Glass transition temperature of a polymer is the temperature at which there is enough thermal energy for the polymer chains to move freely (wiggle around). Differential scanning calorimetry (DSC) method Thermomechanical analysis (TMA) = volume expansion Dynamic Mechanical Analysis (DMA): response to oscillatory shear
- 19. Glass Transition and Melting Point for Thermoplastics
- 20. Glass Transition and Melting https://www.slideshare.net/NikolaiPriezjev
- 21. Thermal Properties of Selected Plastics https://www.slideshare.net/NikolaiPriezjev
- 22. Melting temperature, Tm: Influence of Structure https://www.slideshare.net/NikolaiPriezjev
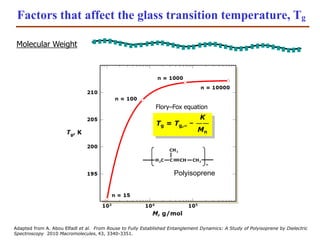
- 23. Factors that affect the glass transition temperature, Tg Molecular Weight Adapted from A. Abou Elfadl et al. From Rouse to Fully Established Entanglement Dynamics: A Study of Polyisoprene by Dielectric Spectroscopy 2010 Macromolecules, 43, 3340-3351. Flory–Fox equation Polyisoprene
- 24. Backbone Flexibility Pendant Groups This backbone is so flexible that polydimethylsiloxane has Tg way down at -127o C! The backbone is very stiff. It's so rigid that it doesn't have a Tg! You can heat it to over 500o C and it will still stay in the glassy state. It will decompose from all the heat before it lets itself undergo a glass transition! Adamantine act like a hook that catches on nearby molecules and keeps the polymer from moving. But big bulky pendant groups can lower the Tg, too. Pendant groups limit how closely the polymer chains can pack together. The further they are from each other, the more easily they can move around. This lowers the Tg, in the same way a plasticizer does. C10H16 Factors that affect the glass transition temperature, Tg
- 25. Factors that affect the glass transition temperature, Tg https://www.slideshare.net/NikolaiPriezjev
- 26. Factors that affect the glass transition temperature, Tg
- 27. Phase Transitions and Structure Tm and Tg increase with • Increasing intermolecular forces • Increasing intra- and intermolecular barriers to chain rotation • Bulky, stiff backbone and side groups • Shorter flexible side groups Adapted from R.F. Boyer, 1963 Rubber Chem. Technol. 36, 1303-1421; L.H. Sperling, 1992 Introduction to Physical Polymer Science, 2nd Ed, pp 303-381.
- 28. Quiz
- 29. Crystallization, Melting, Glass Transition Crystallization: crystalline nuclei form and grow, chains align and order. Crystallization rates can be defined from the same type of S-curves we saw for metals - can be described by the Avrami equation: y = 1 – exp(-k tn) Polypropylene (PP) TH T r m mSL 12 Tm ~160 to 166 °C
- 30. Crystallization, Melting, Glass Transition Dependence of melting and glass transition temperatures and polymer properties on molecular weight.
- 31. Polymer Degradation • Polymer degradation is a change in the properties like tensile strength, color, shape, etc. • Polymer-based product under the influence of one or more environmental factors such as heat, light or chemicals such as acids, alkalis and some salts. Main factors: solar radiation, temperature, moisture, oxygen. These changes are usually undesirable, such as cracking and chemical disintegration of products. Solar radiation: Physical changes resulting from exposure to the environment are initiated by chemical bond breaking reactions caused by the absorbed light. The ultraviolet portion of solar energy, with the shortest wavelengths often having the greatest effect. Solar absorptivity is closely related to color, thus samples of different colors will reach different on-exposure temperatures. https://www.slideshare.net/NikolaiPriezjev
- 32. UV radiation The lower boundary in Earth’s atmosphere solar UV spectrum is caused by ozone shielding. Ozone O3 layer. UV radiation can be classified as near, far or extreme UV but it is also possible to classify UV radiation in terms of UVA, UVB and UVC https://www.slideshare.net/NikolaiPriezjev
- 33. Polymer Degradation (cont.) Photoinduced degradation: • Short wavelength UV radiation causes yellowing; long wavelength UV (penetrate deeper in the material) is primarily responsible for degradation of physical properties, such as tensile strength and impact strength. https://www.slideshare.net/NikolaiPriezjev
- 34. Polymer Degradation (cont.) Thermal degradation: • Chain-growth polymers like poly(methyl methacrylate) PMMA can be degraded by hemolysis at high temperatures to give monomers, oils, gases and water. • For example the PVC eliminates HCl, under 100–120 °C. • CH2(Cl)CHCH2CH(Cl) → CH=CH-CH=CH + 2HCl Hydrochloric acid Biological degradation: • Biodegradable plastics can be biologically degraded by microorganisms to give lower molecular weight molecules. To degrade properly biodegradable polymers need to be treated like compost and not just left in a landfill site where degradation is very difficult due to the lack of oxygen and moisture. • The mechanism of biodegradation is by anaerobic processes, where oxygen is not present.
- 35. Polymer Degradation (cont.) Moisture: Moisture, in combination with solar radiation, contributes significantly to the weathering of many polymeric materials Mechanical stresses imposed when moisture is absorbed or desorbed and to the chemical participation of moisture in the chemical evolution cause weathering The span of time over which the precipitation occurs and the frequency of wetness are important in the weathering of materials Water absorption in the surface layers produces a volume expansion which places mechanical stress on the dry subsurface layers. Drying out of the surface layers would lead to a volume contraction. The hydrated inner layers resist this contraction, leading to surface stress cracking.
- 36. Physical Ageing in Polymers Polymer ageing may involve physical ageing without chemical reaction occurring; chemical changes such as crosslinking during curing of a thermoset; thermal conditioning at elevated temperature; photochemical ageing, as occurs in weathering. Density versus ageing time at room temperature of samples extracted, respectively, from the skin and the core of an injection moulded polystyrene bar. Ageing took place in a density column at 23 °C. Volumetric relaxation after rapid cooling 11days
- 37. • Molecular weight, M: Mass of a mole of chains. Low M high M Not all chains in a polymer are of the same length — i.e., there is a distribution of molecular weights polydispersity Molecular Weight Department of Mechanical and Materials Engineering Wright State University
- 38. Molecular Weight Distribution Most polymers are polydisperse — they contain more than one chain length — i.e., there is a distribution of molecular weights Arrhenius equation )/( RTE Aerate
- 39. Molecular Weight Distribution Molecular weight of ethylene C2H4 = 2 x 12 + 4 x 1 = 28 g/mole Molecular weight of polyethylene 1000 x C2H4 =1000 x 28 g/mole = 28,000 g/mole https://www.slideshare.net/NikolaiPriezjev
- 40. Mass Distribution in Low-MW Polystyrene Adapted from “The Characterization of Polystyrene Oligomers by Field-desorption Mass Spectrometry”, K. Rollins et al., 1990 Rapid Commun. Mass Spectrom., 4, 355-359
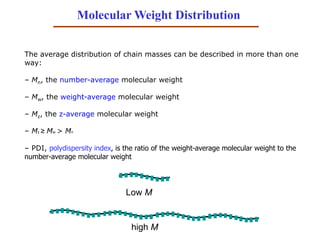
- 41. Molecular Weight Distribution The average distribution of chain masses can be described in more than one way: – Mn, the number-average molecular weight – Mw, the weight-average molecular weight – Mz, the z-average molecular weight – Mz ≥ Mw > Mn – PDI, polydispersity index, is the ratio of the weight-average molecular weight to the number-average molecular weight Low M high M
- 42. Example: average mass of a class Student Weight mass (lb) 1 104 2 116 3 140 4 143 5 180 6 182 7 191 8 220 9 225 10 380 What is the average weight of the students in this class: a) Based on the number fraction of students in each mass range? b) Based on the weight fraction of students in each mass range? Molecular Weight Calculation
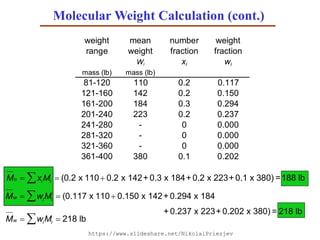
- 43. Solution: The first step is to sort the students into weight ranges. Using 40 lb ranges gives the following table: weight number of mean number weight range students weight fraction fraction Ni Wi xi wi mass (lb) mass (lb) 81-120 2 110 0.2 0.117 121-160 2 142 0.2 0.150 161-200 3 184 0.3 0.294 201-240 2 223 0.2 0.237 241-280 0 - 0 0.000 281-320 0 - 0 0.000 321-360 0 - 0 0.000 361-400 1 380 0.1 0.202 Ni NiWi 10 1881 total number total weight Calculate the number and weight fraction of students in each weight range as follows: xi Ni Ni wi NiWi NiWi For example: for the 81-120 lb range x81120 2 10 0.2 117.0 1881 011x2 12081 w Molecular Weight Calculation (cont.)
- 44. Mn xiMi (0.2 x 110 0.2 x 142+ 0.3 x 184+ 0.2 x 223+ 0.1 x 380) =188 lb weight mean number weight range weight fraction fraction Wi xi wi mass (lb) mass (lb) 81-120 110 0.2 0.117 121-160 142 0.2 0.150 161-200 184 0.3 0.294 201-240 223 0.2 0.237 241-280 - 0 0.000 281-320 - 0 0.000 321-360 - 0 0.000 361-400 380 0.1 0.202 Mw wiMi (0.117 x 110 0.150 x 142+ 0.294 x 184 + 0.237 x 223+ 0.202 x 380) = 218 lb Mw wiMi 218 lb Molecular Weight Calculation (cont.) https://www.slideshare.net/NikolaiPriezjev
- 45. Molecular Weight Distribution Mn: Number-Average Mol. Wgt. The number-average molecular weight (molar mass) of a polymer containing Ni molecules of mass Mi is the arithmetic mean of the molar mass distribution: Mw: Weight-Average Mol. Wgt. The weight-average molecular weight (molar mass) is the sum of the products of the molar mass of each fraction multiplied by its weight fraction (wi). In terms of wi or numbers of molecules, Mw is https://www.slideshare.net/NikolaiPriezjev
- 46. Molecular Weight Distribution Mz: Z-Average Mol. Wgt. The z-average molecular weight (molar mass) is: Mz is especially sensitive to the presence of high-MW chains. PDI: Polydispersity index The molecular weight distribution, or polydispersity index, is the ratio of the weight- average molecular weight to the number-average molecular weight: The polydispersity index of a monodisperse polymer is 1.00. The polydispersity index increases as the polymer distribution broadens.
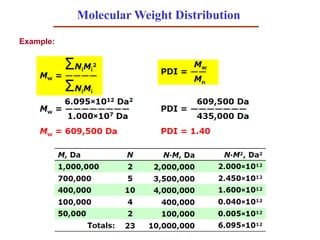
- 47. Molecular Weight Distribution Example: You have a polymer sample that contains the following molecules: What are Mn, Mw, and the polydispersity index? https://www.slideshare.net/NikolaiPriezjev
- 50. Here are: 10 chains of 100 molecular weight 20 chains of 500 molecular weight 40 chains of 1000 molecular weight 5 chains of 10000 molecular weight 1347 5402010 )100005()100040()50020()10010( Mn 5390 )100005()100040()50020()10010( )100005()100040()50020()10010( M 2222 w 4 M M sityPolydisper n w Molecular Weight and Dispersion - an example: Find Mn, Mw, polydispersity index:
- 52. Molecular Weight Distribution Polydispersity index (PDI) is a measure of the distribution of molecular mass in a given polymer sample. PDI=1, many chains with the same length (monodisperse). Otherwise, polydisperse PDI>1. Bimodal MWD 1 Da (Dalton) = 1 g/mol 1 Da = 1.660×10–27 kg Number average: break, yield, and impact strength increase
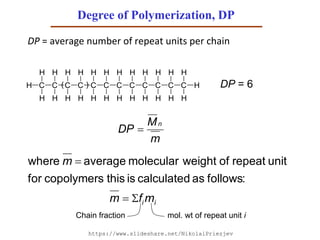
- 53. Degree of Polymerization, DP DP = average number of repeat units per chain ii mfm m :followsascalculatedisthiscopolymersfor unitrepeatofweightmolecularaveragewhere C C C C C C C CH H H H H H H H H H H H H H H H H C C C C H H H H H H H H H( ) DP = 6 mol. wt of repeat unit iChain fraction m M DP n https://www.slideshare.net/NikolaiPriezjev
- 54. Melt Index Simple melt index test T For example: PE 190C/1.0kg Not intrinsic or fundamental property of polymer melt, rather convenient and easy method for expressing flow properties useful for processing. High melt index = low molecular weight; low melt index = high molecular weight. Weighted Plunger Barrel Molten Pellets Extrudate Orifice Heater Band Melt Flow Index # grams of flow per 10 minutes
- 55. Effects of molecular weight, dispersity, branching • The molecular weight, dispersity and branching has a significant effect on the mechanical and physical bulk properties of polymers. In general, a higher molecular weight improves the mechanical properties, that is, break, yield, and impact strength increase. However, a higher molecular weight also increases the melt and glass transition temperature as well as the solution and melt viscosity, which makes processing and forming of the polymeric material more difficult. • The dispersity has the opposite effect; a wider molecular weight distribution lowers the tensile and impact strength but increases the yield strength, or in other words, a lower dispersity (narrower distribution) leads to better mechanical properties. The low-molecular weight portion of the distribution has a similar effect as a plasticizer, that is, it reduces the brittleness and lowers the melt viscosity which improves the processability, whereas the high-molecular weight portion causes processing difficulties because of its huge contribution to the melt viscosity. • Branching is another important performance parameter. In general, branching lowers the mechanical properties. For example, it decreases the break and yield strength. The effect on toughness is less clear; if the length of the branches exceed the entanglement weight it improves the toughness, otherwise it lowers the impact strength. Branching also lowers the brittleness, the melt temperature, the melt and solution viscosity and increases the solubility. In conclusion, the processability improves with increasing degree of branching.
- 56. Shape (Steric) Effects The effects of the shape or size of the atoms or groups of atoms are called steric effects. Various methods for representing the 2- chloropropane (C3H7Cl) molecule: reduced crystallinity => tensile strength, Tg lower. But hindered movement. https://www.slideshare.net/NikolaiPriezjev
- 57. Main physical properties of polymers 1 - Primary bonds: the covalent bonds that connect the atoms of the main chain 2 - Secondary bonds: non – covalent bonds that hold one polymer chain to another including hydrogen bond and van der Waals (dipole –dipole) attraction 3 - Crystalline polymer: solid polymers with high degree of structural order and rigidity 4 - Amorphous polymers: polymers with a low degree of structural order 5 - Semi – crystalline polymer: most polymers actually consist of both crystalline domains and amorphous domains with properties between that expected for a purely crystalline or purely amorphous polymer 6 - Glass: the solid form of an amorphous polymer characterized by rigidity and brittleness 7 – Crystalline melting temperature (Tm): temperature at which crystalline polymers melt 8 - Glass transition temperature (Tg ): temperature at which an amorphous polymer converts to a liquid or amorphous domains of a semi crystalline polymer melt 9 – Thermoplastics (plastics): polymers that undergo thermally reversible conversion between the solid state and the liquid state 10 - Thermosets: polymers that continue reacted at elevated temperatures generating increasing number of crosslinks such polymers do not exhibit melting or glass transition 11 - Liquid–crystalline polymers: polymers with a fluid phase that retains some order 12 - Elastomers: rubbery, stretchy polymers the effect is caused by light crosslinking that pulls the chains back to their original state https://www.slideshare.net/NikolaiPriezjev
- 58. Summary Reading: Chapter 3 of Plastics: Materials and Processing by A. Brent Strong • Gas, liquid, and solid phases, crystalline vs. amorphous structure, viscosity • Thermal expansion and heat distortion temperature • Glass transition temperature, melting temperature, crystallization • Polymer degradation, aging phenomena • Molecular weight distribution, polydispersity index, degree of polymerization • Effects of molecular weight, dispersity, branching on mechanical properties • Melt index, shape (steric) effects