Method Development and Method Validation for the estimation of Valganciclovir in Tablet Formulation by RP- HPLC Method
- 1. Presented by 26103423 Under the supervision of Mr. V. Sivanand, M.Pharm.,Ph.D., Asst Professor, DEPARTMENT OF PHARMACEUTICAL ANALYSIS Ultra college of pharmacy The Tamilnadu Dr.M.G.R Meical University Madurai-20
- 2. INTRODUCTION Analytical Chemistry Analytical chemistry may be defined as the science and art of determining the composition of materials in terms of the elements of compound contained. By means of analytical techniques both qualitative and quantitative analysis can be done. Qualitative methods :- Quantitative analysis:- Classification of analytical methods:- Spectral methods Chromatographic methods Electrochemical methods Miscellaneous methods Hyphenated methods
- 3. CHROMATOGRAPHY Chromatography is a technique used for the separation, purification and identification of the compounds of mixtures by their continuous distribution, between two phases. One is stationary phase and the other is mobile phase. Principles of Chromatographic Separation: Adsorption chromatography: a solid stationary phase and a liquid or gaseous mobile phase. Partition chromatography: a liquid stationary phase and a liquid or gaseous mobile phase. Size exclusion chromatography: an inert gel which acts as a molecular sieve, and liquid mobile phase. Ion exchange chromatography: a solid polymeric stationary phase containing replaceable ions.
- 4. Mode of chromatographic operations: There are three modes of chromatographic operation they are as follows: 1. Elution techniques Isocratic method Gradient method 2. Frontal techniques 3. Displacement techniques Types of chromatography techniques: Planar chromatography Column chromatography
- 5. TYPES OF LIQUID CHROMATOGRAPHY:- Basic operation of Liquid chromatography 1. Feed Injection: 2. Separation in the column: 3. Elution from the column: 4. Detection: High performance liquid chromatography – [HPLC] Different types of HPLC Techniques 1. Normal phase chromatography. 2. Reverse phase chromatography. 3. Size exclusion chromatography. 4. Ion exchange chromatography.
- 6. INSTRUMENTATION OF HPLC Mobile phase Pumps Injector port Stationary phase Detector ANALYTICAL METHOD DEVELOPMENT Selecting an accurate assay procedure for each ingredient present in pharmaceutical dosage forms, either individually or complex dosage formulation containing several therapeutically and chemically compatible drugs with very similar chemical nature is a monumental undertaking.
- 7. STEPS TO BE FOLLOWED IN METHOD DEVELOPMENT 1. Standard Analyte Characterization 2. Method Requirements 3. Literature Search and prior Methodology 4. Choosing of Method 5. Instrumental Setup and Initial Studies 6. Optimization 7. Documentation of analytical figures 8. Evaluation of Method Development with actual Sample 9. Determination of Percent Recovery of Actual Sample and Demonstration of Quantitative Sample Analysis
- 8. VALIDATION OF ANALYTICAL METHOD DEVELOPMENT Analytical method validation is the process of demonstrating that analytical procedures are suitable for their intended use and provide accurate test results that evaluate a product against its defind specification and quality attributes. VALIDATION OF ANALYTICAL PROCEDURES Different Types of Validation characteristics: A. Precision B. Accuracy C. Specificity and Selectivity D. Linearity and Range E. Forced degradation Studies F. Limit of Detection (LOD) G. Limit of Quantification (LOQ) H. Robustness I. Ruggedness. J. System Suitability
- 9. VALIDATION OF ANALYTICAL PROCEDURES Different Types of Validation characteristics: A. Precision B. Accuracy C. Specificity and Selectivity D. Linearity and Range E. Forced degradation Studies F. Limit of Detection (LOD) G. Limit of Quantification (LOQ) H. Robustness I. Ruggedness. J. System Suitability
- 10. REVIEW OF LITERATURE Baburao et al., reported a simple sensitive and economical UV spectrophotometric method for the determination of valganciclovir in bulk and tablet dosage form. Valganciclovir shows maximum absorbance at 254nm in methanol. Beer’s law was obeyed within the concentration range of 5-30 mcg/ml with the correlation coefficient of 0.9999. The standard plot was clearly showed a straight line passing through the origin. The method was extended to pharmaceutical formulations. B.dagontopa et al., reported a rapid, sensitive, and specific reverse phase high performance liquid chromatography with diode array detection procedure for the simultaneous determination of abacavir, efavirenz and valganciclovir in spiked human serum is described. Separation was performed on a 5µm waters spherisorb column (256 4.6 mm ID) with acetonitrile: methanol: KH2PO4 (at PH 5.0) (40:20:40 v/v/v) isocratic elution at a flow rate of 1.0 ml min-1. Calibration curves were constructed in the range of 50-30,000 ng mL-1 for abacavir and efavirenz, and 10-30,000 ng mL-1 for serum samples. The limit of detection and limit of quantification concentration of the HPLC method were 3.80 and 12.68 ng mL-1 for abacavir, 2.61 and 8.69 ng mL-1 for efavirenz, 1.30 and 4.32 ng ml-1 for valganciclovir. The method for the simultaneous determination of these three compounds in human serum.
- 11. DRUG PROFILE VALGANCICLOVIR Structural formula Common Name : Valganciclovir Chemical Name IUPAC : 2-[(2-amino-6,9-dihydro-3H-purin-9-yl)methoxy]-3- hydroxy propyl(2s)-2-amino-3-methyl butanoate. Empirical formula : C14H22N6O5.HCL Molecular weight : 390.83 Nature : White crystalline powder Solubility : Soluble in methanol, slightly soluble in water Melting point : 1800C Purity : 98.0% to 102.0% Category : Anti viral, Anti-Herpes virus
- 12. INSTRUMENTS AND REAGENTS Instruments: S.No Name of the Make Model Instrument 1. HPLC Water Alliance-2695 PDA Waters-2996 2. Electronic Shimadzu AY 220 balance 3. Digital PH meter Digisun 7007 Electronics 4. Centrifuge Thermolab R8C 5. PhotoStability Thermolab 943/03/0607 Chamber
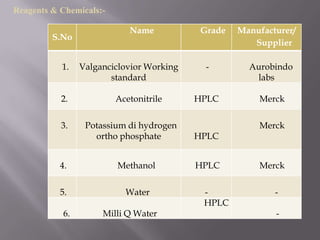
- 13. Reagents & Chemicals:- Name Grade Manufacturer/ S.No Supplier 1. Valganciclovior Working - Aurobindo standard labs 2. Acetonitrile HPLC Merck 3. Potassium di hydrogen Merck ortho phosphate HPLC 4. Methanol HPLC Merck 5. Water - - HPLC 6. Milli Q Water -
- 14. OBJECTIVE AND PLAN OF THE WORK The literature survey indicates that are some methods reported for the determination of valganciclovir in human plasma and in combination with other drugs like penciclovir, ganciclovir, aciclovir by LC-MS, ESI-MS/MS, Fluorescence, HPLC and UV with longer quantitation time. The aim of present work is to develop and validate simple RPHPLC method by isocratic mode for the quantification of valganciclovir in bulk and it’s formulation.
- 15. METHOD DEVELOPMENT AND OPTIMIZATION 1. Selection of wave length: S. No. Wavelength Absorbance 1. 234 0.549 2. 240 1.014 3. 246 1.469 4. 252 1.821 5. 254 2.047 6. 264 1.936
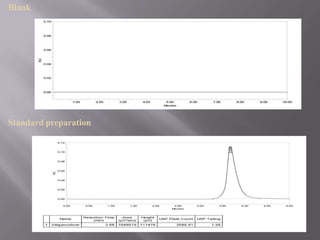
- 16. Optimization of chromatographic parameters: a. selection of mode of separation. b. Selection and standardization of mobile phase and column Standard solution of valganciclovir:- FIXED CHROMATOGRAPHIC CONDITION Instrument: waters 2695 separations module, UV- 2998 PDA detector Column : Symmetry C8 (4.6 150, 15µm) Wavelength: 254 nm Temperature: Ambient temperature (250C) Flow rate: 0.6ml/min Injection: 20µl Mobile phase: Acetonitrile: Phosphate buffer (pH4) (45:55) Retention time: Valganciclovir – 3.68
- 18. QUANTITATIVE DETERMINATION OF THE DRUG BY USING THE DEVELOPED METHOD Sample: Valganciclovir Label claim: 450mg Sample solution of valganciclovir:- Amount of drug present in the tablet: Sample area Standard dilution ------------------ x --------------------- x Average weight of tablet Standard area Sample dilution Amount present Percentage content = ----------------------- x 100 Label claim
- 20. Sample solution Quantitative Estimation S.No Brand content Label Peakarea Amount Percentage Name Claim(mg) Present(mg) Content(%) 1. Valcyte Valganciclovir 450mg 1648428 440mg 98.6% Acceptance criteria: 98.0- 102%w/v.
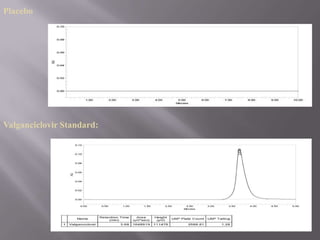
- 21. SPECIFICITY The specificity of an analytical method is its ability to measure accurately and specifically the analytes in the presence of compounds that may be expected to be present in the sample matrix. Blank
- 23. Valganciclovir standard + placebo Specificity for valganciclovir S.No Sample Area obtained Percentage content of Drug 1. Placebo 0 0 2. Standard 1648919 98.6% 3. Standard+Placebo 1631852 99.5%
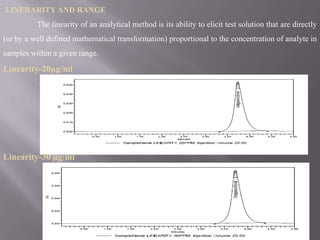
- 24. LINERARITY AND RANGE The linearity of an analytical method is its ability to elicit test solution that are directly (or by a well defined mathematical transformation) proportional to the concentration of analyte in samples within a given range. Linearity-20µg/ml Linearity-30 µg/ml
- 26. Linearity-60 µg/ml STANDARD LINEARITY OF VAGANCICLOVIR
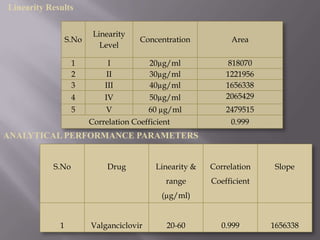
- 27. Linearity Results Linearity S.No Concentration Area Level 1 I 20µg/ml 818070 2 II 30µg/ml 1221956 3 III 40µg/ml 1656338 4 IV 50µg/ml 2065429 5 V 60 µg/ml 2479515 Correlation Coefficient 0.999 ANALYTICAL PERFORMANCE PARAMETERS S.No Drug Linearity & Correlation Slope range Coefficient (µg/ml) 1 Valganciclovir 20-60 0.999 1656338
- 28. ACCURACY :-The accuracy of an analytical method is the closeness of that results obtained by that method to the true value. Accuracy may often be expressed as percent recovery by the assay of known added amount of analyte. Standard preparation Valganciclovir 50% solution-
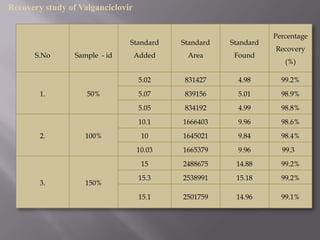
- 32. Recovery study of Valganciclovir Percentage Standard Standard Standard Recovery S.No Sample - id Added Area Found (%) 5.02 831427 4.98 99.2% 1. 50% 5.07 839156 5.01 98.9% 5.05 834192 4.99 98.8% 10.1 1666403 9.96 98.6% 2. 100% 10 1645021 9.84 98.4% 10.03 1665379 9.96 99.3 15 2488675 14.88 99.2% 15.3 2538991 15.18 99.2% 3. 150% 15.1 2501759 14.96 99.1%
- 33. PRECISION Precision of an analytical method is the degree of agreement among individual test results when the procedure is applied repeatedly to multiple sampling of a homogenous sample precision of analytical method is usually expressed as the standard deviation and relative standard deviation. A. System Precision B. Method precision
- 34. System precision
- 36. System precision data Injection Area Injection-1 1642147 Injection-2 1676409 Injection-3 1676588 Injection-4 1670567 Injection-5 1676215 Injection-6 1672426 Average 1669058 Standard Deviation 13415 %RSD 0.81
- 37. Method Presicion
- 39. Method precision of Valganciclovir S.No Area Obtained Amount present Percentage (m.g) Content(%) 1. 1665599 9.96 99.6 2. 1657621 9.91 99.1 3. 1644289 9.85 98.2 4. 1660606 9.93 99.3 5. 1661522 9.93 99.3 6. 1653829 9.89 98.9 MEAN 99.06 STANDARD DEVIATION 0.4844 . RELATIVE STANDARD DEVITATION 0.488
- 40. Limit of Detection: (LOD) It is the lowest amount of analyte in a sample that can be detected but not necessarily quantities as an exact value under the stated, experimental conclusions. The detection limit is usually expressed as the concentration of analyte. The Standard deviation of the response and the slope 3.3 X Standard deviation (σ) LOD= S S= slope of the calibration curve of the analyte. 3.3 X 658793 = 1656338 = 1.31
- 41. Limit of Quantitation: (LOQ) The quantitation limit of an analytical procedure is the lowest amount of analyte in a sample which can be quantitatively determined with suitable precision and accuracy. The Standard deviation of the response and the slope 10 X Standard deviation (σ) LOQ = S S= slope of the calibration curve of the analyte. 10 X 658793 = 1656338 = 3.97
- 42. RUGGEDNESS Ruggedness as the degree of reproducibility of test result obtained by the analysis of the same of the samples under verity of normal test conditions, such as different labs, different analysis, and different lots of reagents, different elapsed assay times, different assay temperature, different days etc.
- 45. RUGGEDNESS OF THE METHOD S.No Column Instrument Analyst Result code Code Obtained (%) 1. C-01 W-29 I 100.1% 2. C-02 W-30 II 99.6% 3. C-03 W-31 III 99.8% Parameter Result observed Acceptance Criteria %Content 99.83 98 – 102%
- 46. ROBUSTNESS It is a measure of ability to remain unaffected by small but deliberate variations in method parameters and provides an indication of its reliability during normal usage. Robustness floe rate: 0.4ml/min Robustness flow rate: 0.6ml/min
- 47. Robustness flow rate: 0.8ml/min System Suitability Results Flow Rate Sample S.No USP Plate Count USP Tailing (ml/min) area 1 0.4 1632631 2301.20 1.21 2 0.6 1645347 2649.91 1.26 3 0.8 1621840 2125.70 1.11 * Results for actual flow (0.6ml/min) have been considered from Assay standard.
- 48. ROBUSTNESS MOBILE PHASE (44:56) ROBUSTNESS MOBILE PHASE (45:55):
- 49. ROBUSTNESS MOBILE PHASE (46:54) System Suitability Results Change in Organic Sample S.No Composition in the area USP Plate Count USP Tailing Mobile Phase 1 44:56 1637097 2142.56 1.08 2 45:55 1645347 2649.91 1.26 3 46:54 1623206 2179.15 1.20 Results for actual Mobile phase composition (45:55 Acetonitrile: Buffer) have been considered.
- 50. SYSTEM SUITABILTY PARAMETERS System suitability testing is an integral part of many analytical procedures. The tests are based on the equipment, electronics, analytical operation and sample to be analyzed constitute an integral system that can be evaluated as such. System suitability test parameters to be established for a particular procedure depend on the procedure type being validated.
- 52. System Suitability Parameters: Parameters Results Tailing factor 1.28 %RSD 0.89 Number of Theoretical 2588.81 plates
- 53. RESULTS & DISCUSSION
- 54. Method development: The developed method has an advantage of determination of valganciclovir in RP-HPLC. The following tables give the results of the method development, quantitation and validation parameters. FIXED CHROMATOGRAPHIC CONDITION Instrument: waters 2695 separations module, UV- 2998 PDA detector Column : Symmetry C8 (4.6 150, 15µm) Wavelength: 254 nm Temperature: Ambient temperature (250C) Flow rate: 0.6ml/min Injection: 20µl Mobile phase: Acetonitrile: Phosphate buffer (pH4) (45:55) Retention time: Valganciclovir – 3.71
- 55. Quantitative Estimation . S. No. Content Label claim Peak area Amount Percentage purity (mg) present (mg) 1. Valganciclovir 450mg 1648428 440mg 98.6% Acceptance criteria: 98– 102% w/v
- 56. HPLC S.No Parameters result Acceptance Criteria 1. SPECIFICITY 99.5% No interference of excipients 2. LINEARITY 0.999 0.99 3. LOD 1.31µg/ml - 4. LOQ 3.97µg/ml - 5. ACCURACY 98.9% 98 – 102% PRECISION 6. System Precision 0.81% 2% (RSD) Method Precision 0.488% 2% (RSD) 7. RUGGEDNESS 99.83% 98-102% 0.815% 2% (RSD) 8. ROBUSTNESS a)Change in flow rate 98 – 102% (+0.2 ml/min) 100.1% (-0.2 ml/min) 99.3% b)Change in mobile phase 98– 102% (44:56) 99.1% (46:54) 98% System suitability 9. a)Theoritical Plates 2588.81 NLT 2000 b)Taling factor 1.16 NMT 2 c)RSD% 0.89 NMT 2.0%
- 57. CONCLUSION





![TYPES OF LIQUID CHROMATOGRAPHY:-
Basic operation of Liquid chromatography
1. Feed Injection:
2. Separation in the column:
3. Elution from the column:
4. Detection:
High performance liquid chromatography – [HPLC]
Different types of HPLC Techniques
1. Normal phase chromatography.
2. Reverse phase chromatography.
3. Size exclusion chromatography.
4. Ion exchange chromatography.](https://image.slidesharecdn.com/presentation1-120713052907-phpapp01/85/Method-Development-and-Method-Validation-for-the-estimation-of-Valganciclovir-in-Tablet-Formulation-by-RP-HPLC-Method-5-320.jpg)





![DRUG PROFILE
VALGANCICLOVIR
Structural formula
Common Name : Valganciclovir
Chemical Name IUPAC : 2-[(2-amino-6,9-dihydro-3H-purin-9-yl)methoxy]-3-
hydroxy propyl(2s)-2-amino-3-methyl butanoate.
Empirical formula : C14H22N6O5.HCL
Molecular weight : 390.83
Nature : White crystalline powder
Solubility : Soluble in methanol, slightly soluble in water
Melting point : 1800C
Purity : 98.0% to 102.0%
Category : Anti viral, Anti-Herpes virus](https://image.slidesharecdn.com/presentation1-120713052907-phpapp01/85/Method-Development-and-Method-Validation-for-the-estimation-of-Valganciclovir-in-Tablet-Formulation-by-RP-HPLC-Method-11-320.jpg)