Analytical QbD
- 2. Introduction Analytical Quality by Design (AQbD) Implementation of AQbD- Practical aspects Case study Conclusion References Dept. of Quality Assurance, DLHHCOP 2
- 3. 3Dept. of Quality Assurance, DLHHCOP Introduction
- 4. Dept. of Quality Assurance, DLHHCOP 4 AQbD- Key components
- 5. Role of analytical methods in drug developmentRole of analytical methods in drug development processprocess 5Dept. of Quality Assurance, DLHHCOP AQbD- Drug Development Process
- 6. Dept. of Quality Assurance, DLHHCOP 6 AQbD- Benefits
- 7. 7Dept. of Quality Assurance, DLHHCOP Traditional versus AQbD
- 8. Steps Synthetic development (QbD) Analytical development (AQbD) 1 QTPP identification ATP (Analytical Target Profile) identification 2 CQA/CMA identification, Risk Assessment CQA identification, Initial Risk Assessment 3 Define product design space with DoE Method Optimization and development with DOE 4 Refine product design space MODR (Method Operable Design Region) 5 Control Strategy with Risk Assessment Control Strategy with Risk Assessment 6 Process validation AQbD Method Validation 7 Continuous process Monitoring Continuous process Monitoring QbD tools for synthetic development and analytical development. 8Dept. of Quality Assurance, DLHHCOP Traditional versus AQbD
- 9. 9Dept. of Quality Assurance, DLHHCOP AQbD Workflow
- 10. Analytical Target Profile (ATP) Analytical Method Performance Characteristics S. No. Method performance characteristics Defined by ICH and USP 1 Accuracy, specificity, and linearity Systematic variability 2 Precision, detection limit, and quantification limit Inherent random variability 3 Range and robustness Not applicable 10Dept. of Quality Assurance, DLHHCOP AQbD Practical Aspects
- 11. Selection of Analytical Techniques Risk Assessment Design of Experiments (DoE) › Screening › Optimization › Selection of DOE Tools › Method Operable Design Region (MODR) and Surface Plots › Model Validation Risk factor = Severity × Occurrence × Detestability 11Dept. of Quality Assurance, DLHHCOP AQbD Practical Aspects
- 12. Design of Experiments (DoE) › Screening, Optimization and Selection of DoE tools Design Number of variables and usage Advantage Disadvantage Full factorial design Optimization/2–5 variables Identifying the main and interaction effect without any confounding Experimental runs increase with increase in number of variables Fractional factorial design or Taguchi methods Optimization/and screening variables Requiring lower number of experimental runs Resolving confounding effects of interactions is a difficult job Plackett-Burman method Screening/or identifying vital few factors from large number of variables Requiring very few runs for large number of variables It does not reveal interaction effect Pseudo-Monte Carlo sampling (pseudorandom sampling) method Quantitative risk analysis/optimization Behaviour and changes to the model can be investigated with great ease and speed. This is preferred where exact calculation is possible For nonconvex design spaces, this method of sampling can be more difficult to employ. Random numbers that can be produced from a random number generating algorithm Full factorial design Optimization/ 2–5 variables Identifying the main and interaction effect without any confounding Experimental runs increase with increase in number of variables 12Dept. of Quality Assurance, DLHHCOP AQbD Practical Aspects
- 13. › Method Operable Design Region (MODR) and Surface Plot › Model Validation Contour plot for MODR Systematic simulation graph for retention time (X2-axis) as method response at constant X3 (0.8 mL/min as flow rate) with change in pH (X1--axis). (Graph shows significant correlation between the predicted retention time and actual (experimental) retention time with good correlation coefficient. 13Dept. of Quality Assurance, DLHHCOP Method Operable Design Region (MODR) and Surface Plot Model Validation AQbD Practical Aspects
- 14. Method Verification/Validation Control Strategy- Continuous Method Monitoring 14Dept. of Quality Assurance, DLHHCOP AQbD Practical Aspects S. No. Pharmaceutic al testing Control strategy 1 Raw material testing Specification based on product QTPP and CQA Effects of variability, including supplier variations, on process and method development are understood 2 In-process testing Real time (at-, on-, or in-line) measurements Active control of process to minimize product variation Criteria based on multivariate process understanding 3 Release testing Quality attributes predictable from process inputs (design space)Specification is only part of the quality control strategy Specification based on patient needs (quality, safety, efficacy, and performance) 4 Stability testing Predictive models at release minimize stability failures Specification set on desired product performance with time
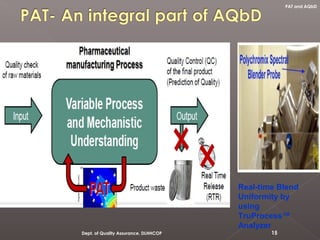
- 15. Real-time Blend Uniformity by using TruProcess™ Analyzer 15Dept. of Quality Assurance, DLHHCOP PAT and AQbD
- 16. Analytical Quality by Design Approach in RP-HPLC Method Development for the Assay of Etofenamate in Dosage Forms Step 1: Target measurement 16Dept. of Quality Assurance, DLHHCOP AQbD- Case Study
- 17. Step 2: DoE:Design of Experiment (Method Optimization and Development) 17Dept. of Quality Assurance, DLHHCOP Experimental Design AQbD- Case Study
- 18. Step 3: Method Operable Design Region pH of aqueous phase versus % of aqueous phase contour at 1.2ml/min flow rate of mobile phase 18Dept. of Quality Assurance, DLHHCOP AQbD- Case Study
- 19. Quadratic model was obtained on application of SigmaTech software with the polynomial equation: Y=5.8778-0.0025X1+2.9925X2–0.8088X3–0.4925X1X2 0.075X1X3-0.125X2X3+0.1178X12 +1.1803X22+0.2768X32 19Dept. of Quality Assurance, DLHHCOP Step 4: DoE: Model validation using regression analysis Developed Chromatogram AQbD- Case Study
- 20. 20Dept. of Quality Assurance, DLHHCOP Step 5: : Method validation AQbD- Case Study
- 21. In a nutshell…… Parameter Traditional Product QbD AQbD Approach Based on empirical approach Based on systematic approach Based on systematic approach Quality Quality is assured by end product testing Quality is built in the product and process by design and scientific approach Robustness and reproducibility of the method built in method development stage FDA submission Including only data for submission Submission with product knowledge and process understanding Submission with product knowledge and assuring by analytical target profile Specifications Specifications are based on batch history Specifications are based on product performance requirements Based on method performance to ATP criteria Process Process is frozen and discourages changes Flexible process with design space allows continuous improvement Method flexibility with MODR and allowing continuous improvement Targeted response Focusing on reproducibility, ignoring variation Focusing on robustness which understands control variation Focus on robust and cost effective method Advantage Limited and simple It is expended process analytical technology (PAT) tool that replaces the need for end product testing Replacing the need of revalidation and minimizing OOT and OOS 21Dept. of Quality Assurance, DLHHCOP AQbD- Summary
- 22. Dept. of Quality Assurance, DLHHCOP 22 %ofresearch AQbD- Summary
- 23. AQbD requires the right ATP and Risk Assessment and usage of right tools and performing the appropriate quantity of work within proper timelines. ‘RIGHT ANALYTICS AT THE RIGHT TIME’ 23Dept. of Quality Assurance, DLHHCOP AQbD- Conclusion
- 24. Raman, N. V. V. S. S.; Mallu, U. R.; Bapatu, H. R. J. Chem.2014, 2015 (1), 8. Torbeck L. D.J. Pharm.Tech.35 (10), 2011,46–47 ICH Harmon. Tripart. Guidel. 2009, 8 (August), 1–28. Jackson, P. 2013, Technical note, http://www.gmpcompliance.org/daten/training/ECA_QbD_in_Analysis_2013 (accessed Oct 23, 2016). Warf S. F. 2013, Conference note; http:// www.ISPE.org/2013QbDConference (accessed Oct 23, 2016). Jadhav, M. L.; Tambe, S. R. Chromatogr. Res. Int. 2013, 2013 (2), 1–9. Borman, P.; Roberts, J.; Jones, C.; Hanna-Brown, M.; Szucs, R.; Bale, nd S. 2010, 2 (7), 2–4. Hanna-brown, M.; Borman, P.; Bale, S.; Szucs, R. Sep. Sci. 2010, 2, 12–20. Nethercote P.; Borman P.; Bennett T.; Martin G.; McGregor P. 2010, 1–9. Vogt, F. G.; Kord, A. S. Pharm. Sci. 2011, 100 (3), 797–812. Bhatt, D. A.; Rane, S. I. Int. J. Pharm. Pharm. Sci. 2011, 3 (1), 179–187. Swartz, M.; Lukulay, P. H.; Krull, I.; Joseph, T. LCGC North Am. 2008, 26 (12), 1190–1197. Meyer, C.; Soldo,T.; Kettenring, U. Chim. Int. J. Chem. 2010, 64 (11), 825–825. McBrien, M. A.; Ling, S.. The Column 2011, 7 (5), 16–20. Molnár, I.; Rieger, H. J.; Monks, K. E. J. Chromatogr. A 2010, 1217 (19), 3193–3200. Karmarkar, S.; Garber, R.; Genchanok, Y.; George, S.; Yang, X.; Hammond, R. J. Chromatogr. Sci. 2011, 49 (6), 439–446.. Monks, K. E.; Rieger, H.-J.; Molnár, I. J. Pharm. Biomed. Anal. 2011, 56 (5), 874–879. Reid G. L., Cheng G., Fortin et al D. T. J. Liq. Chromatogr. Relat. Tecnhologies 2013, 36 (18), 2612–2638. Dept. of Quality Assurance, DLHHCOP 24 References
- 25. Monks, K.; Molnár, I.; Rieger, H. J.; Bogáti, B.; Szabó, E. J. Chromatogr. A 2012, 1232, 218–230. Orlandini, S.; Pinzauti, S.; Furlanetto, S. Anal. Bioanal. Chem. 2013, 405 (2–3), 443–450. Musters, J.; Van Den Bos, L.; Kellenbach, E. Org. Process Res. Dev. 2013, 17 (1), 87–96. Xavier, C. M.; Basavaiah, K.; Vinay, K. B.; Swamy, N. ISRN Chromatogr. 2013, 2013, 1–10.. Xavier, C. M.; Basavaiah, K.; Xavier, C. M.; Basavaiah, K. ISRN Chromatogr. 2012, 2012, 1–11. Dasare, P. http://sspcmsn.org/yahoo_site_admin/assets/docs/Analytical_approach_in_QbD_SSPC.4416180 8.pdf (accessed on Oct 26, 2016). Chatterjee, S. IFPAC Annu. Meet.2013 Morgado, J.; Barnett, K.; Ph, D.; Harrington, B.; Wang, J.; Ph, D.; Harwood, J. 2013, 2, 1–14. Elder, D.; Borman, P. Pharm. Outsourcing 2013. Zlota, A. A.; Zlota, T.; Llc, C. 2014.. ASME. 2010, https://www.asme.org/products/codesstandards/b89731-2001guidelines decision- rules-considering (accessed on Oct 26, 2016). Guide, C.; Edition, F. Interpret. A J. Bible Theol. 2007, 18. Burnett K. L., Harrington B., and Graul T. W. 2013. Jadhav, M. L.; Tambe, S. R. Chromatogr. Res. Int. 2013, 2013, 1–9. ICH Expert Working Group. ICH Harmon. Tripart. Guidel. 2005, No. November, 1–23. http://www.ssciinc.com/DrugSubstance/PATandPharmaceuticalQualityByDesign/ tabid/86/Default.aspx (accessed on Oct 26, 2016). Patel, G. M.; Shelat, P. K.; Lalwani, A. N. Eur. J. Pharm. Sci.2016. Li, Y.; Liu, D. Q.; Yang, S.; Sudini, R.; McGuire, M. A.; Bhanushali, D. S.; Kord, A. S. J. Pharm. Biomed. Anal.2010, 52 (4), 493–507. Dept. of Quality Assurance, DLHHCOP 25 References
- 26. 26Dept. of Quality Assurance, DLHHCOP