Clay Minerals And Soil Structure
- 1. IV Clay Minerals and Soil Structure
- 2. Outline Clay Minerals Identification of Clay Minerals Specific Surface (S s ) Interaction of Water and Clay Minerals Interaction of Clay Particles Soil Structure and Fabric Soil Fabric-Natural Soil Soil Fabric-Clay Soils Soil Fabrics-Granular Soils Loess Suggested Homework References
- 4. 1.1 Origin of Clay Minerals “ The contact of rocks and water produces clays, either at or near the surface of the earth” (from Velde, 1995) . Rock +Water Clay For example, The CO 2 gas can dissolve in water and form carbonic acid, which will become hydrogen ions H + and bicarbonate ions, and make water slightly acidic. CO 2 +H 2 O H 2 CO 3 H + +HCO 3 - The acidic water will react with the rock surfaces and tend to dissolve the K ion and silica from the feldspar. Finally, the feldspar is transformed into kaolinite. Feldspar + hydrogen ions+water clay (kaolinite) + cations, dissolved silica 2KAlSi 3 O 8 +2H + +H 2 O Al 2 Si 2 O 5 (OH) 4 + 2K + +4SiO 2 Note that the hydrogen ion displaces the cations.
- 5. 1.1 Origin of Clay Minerals (Cont.) The alternation of feldspar into kaolinite is very common in the decomposed granite. The clay minerals are common in the filling materials of joints and faults (fault gouge, seam) in the rock mass. Weak plane!
- 6. 1.2 Basic Unit-Silica Tetrahedra Hexagonal hole 1 Si 4 O (Si 2 O 10 ) -4 Replace four Oxygen with hydroxyls or combine with positive union (Holtz and Kovacs, 1981) Tetrahedron Plural: Tetrahedra
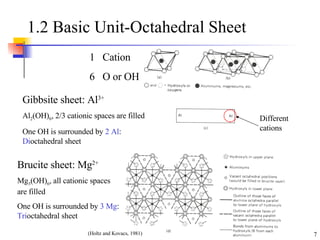
- 7. 1.2 Basic Unit-Octahedral Sheet Gibbsite sheet: Al 3+ Al 2 (OH) 6 , 2/3 cationic spaces are filled One OH is surrounded by 2 Al : Di octahedral sheet Brucite sheet: Mg 2+ Mg 3 (OH) 6 , all cationic spaces are filled One OH is surrounded by 3 Mg : Tri octahedral sheet Different cations 1 Cation 6 O or OH (Holtz and Kovacs, 1981)
- 8. 1.2 Basic Unit-Summary Mitchell, 1993
- 9. 1.3 Synthesis Noncrystalline clay -allophane Mitchell, 1993
- 10. 1.4 1:1 Minerals-Kaolinite Basal spacing is 7.2 Å Si 4 Al 4 O 10 (OH) 8 . Platy shape The bonding between layers are van der Waals forces and hydrogen bonds (strong bonding). There is no interlayer swelling Width: 0.1~ 4 m, Thickness: 0.05~2 m layer Trovey, 1971 ( from Mitchell, 1993) 17 m
- 11. 1.4 1:1 Minerals-Halloysite Si 4 Al 4 O 10 (OH) 8 · 4H 2 O A single layer of water between unit layers. The basal spacing is 10.1 Å for hydrated halloysite and 7.2 Å for dehydrated halloysite. If the temperature is over 50 ° C or the relative humidity is lower than 50%, the hydrated halloysite will lose its interlayer water (Irfan, 1966) . Note that this process is irreversible and will affect the results of soil classifications (GSD and Atterberg limits) and compaction tests. There is no interlayer swelling. Tubular shape while it is hydrated. Trovey, 1971 ( from Mitchell, 1993) 2 m
- 12. 1.5 2:1 Minerals-Montmorillonite n ·H 2 O+cations 5 m Si 8 Al 4 O 20 (OH) 4 ·nH 2 O (Theoretical unsubstituted). Film-like shape. There is extensive isomorphous substitution for silicon and aluminum by other cations, which results in charge deficiencies of clay particles. n · H 2 O and cations exist between unit layers, and the basal spacing is from 9.6 Å to (after swelling) . The interlayer bonding is by van der Waals forces and by cations which balance charge deficiencies (weak bonding). There exists interlayer swelling, which is very important to engineering practice ( expansive clay ). Width: 1 or 2 m, Thickness: 10 Å ~1/100 width (Holtz and Kovacs, 1981)
- 13. 1.5 2:1 Minerals-Illite (mica-like minerals) potassium Si 8 (Al,Mg, Fe) 4~6 O 20 (OH) 4 ·(K,H 2 O) 2 . Flaky shape. The basic structure is very similar to the mica, so it is sometimes referred to as hydrous mica. Illite is the chief constituent in many shales. Some of the Si 4+ in the tetrahedral sheet are replaced by the Al 3+ , and some of the Al 3+ in the octahedral sheet are substituted by the Mg 2+ or Fe 3+ . Those are the origins of charge deficiencies. The charge deficiency is balanced by the potassium ion between layers. Note that the potassium atom can exactly fit into the hexagonal hole in the tetrahedral sheet and form a strong interlayer bonding. The basal spacing is fixed at 10 Å in the presence of polar liquids (no interlayer swelling). Width: 0.1~ several m, Thickness: ~ 30 Å 7.5 m Trovey, 1971 ( from Mitchell, 1993) K
- 14. 1.5 2:1 Minerals-Vermiculite (micalike minerals) The octahedral sheet is brucite. The basal spacing is from 10 Å to 14 Å. It contains exchangeable cations such as Ca 2+ and Mg 2+ and two layers of water within interlayers. It can be an excellent insulation material after dehydrated. Illite Vermiculite Mitchell, 1993
- 15. 1.6 2:1:1 Minerals-Chlorite The basal spacing is fixed at 14 Å. Gibbsite or brucite
- 16. 1.7 Chain Structure Clay Minerals They have lathlike or threadlike morphologies. The particle diameters are from 50 to 100 Å and the length is up to 4 to 5 m. Attapulgite is useful as a drilling mud in saline environment due to its high stability. Attapulgite 4.7 m Trovey, 1971 ( from Mitchell, 1993)
- 17. 1.8 Mixed Layer Clays Different types of clay minerals have similar structures (tetrahedral and octahedral sheets) so that interstratification of layers of different clay minerals can be observed. In general, the mixed layer clays are composed of interstratification of expanded water-bearing layers and non-water-bearing layers. Montmorillonite-illite is most common, and chlorite-vermiculite and chlorite-montmorillonite are often found. (Mitchell, 1993)
- 18. 1.9 Noncrystalline Clay Materials Allophane Allophane is X-ray amorphous and has no definite composition or shape. It is composed of hollow, irregular spherical particles with diameters of 3.5 to 5.0 nm.
- 19. 2. Identification of Clay Minerals
- 20. 2.1 X-ray diffraction The distance of atomic planes d can be determined based on the Bragg’s equation. BC+CD = n , n = 2d · sin , d = n /2 sin where n is an integer and is the wavelength. Different clays minerals have various basal spacing (atomic planes). For example, the basing spacing of kaolinite is 7.2 Å. Mitchell, 1993
- 21. 2.2 Differential Thermal Analysis (DTA) For example: Quartz changes from the to form at 573 º C and an endothermic peak can be observed. Differential thermal analysis (DTA) consists of simultaneously heating a test sample and a thermally inert substance at constant rate (usually about 10 º C/min) to over 1000 º C and continuously measuring differences in temperature and the inert material T. Endothermic (take up heat) or exothermic (liberate heat) reactions can take place at different heating temperatures. The mineral types can be characterized based on those signatures shown in the left figure. (from Mitchell, 1993) T Temperature (100 º C)
- 22. 2.2 DTA (Cont.) If the sample is thermally inert , If the phase transition of the sample occurs, T Time t T Time t Crystallize Melt Endothermic reactions take up heat from surroundings and therefore the temperature T decreases. Exothermic reactions liberate heat to surroundings and therefore the temperature T increases . T= the temperature of the sample – the temperature of the thermally inert substance.
- 23. 2.3 Other Methods Electron microscopy Specific surface (S s ) Cation exchange capacity (cec) Plasticity chart
- 24. 2.3 Other Methods (Cont.) 5. Potassium determination Well-organized 10Å illite layers contain 9% ~ 10 % K 2 O. 6. Thermogravimetric analysis It is based on changes in weight caused by loss of water or CO2 or gain in oxygen. Sometimes, you cannot identify clay minerals only based on one method.
- 25. 3. Specific Surface (S s )
- 26. 3.1 Definition Example: Surface related forces: van der Waals forces, capillary forces, etc. S s is inversely proportional to the particle size Preferred
- 27. 3.2 Typical Values Montmorillonite Illite Kaolinite 50-120 m 2 /gm (external surface) 700-840 m 2 /gm (including the interlayer surface) 65-100 m 2 /gm 10-20 m 2 /gm Interlayer surface
- 28. 4. Interaction of Water and Clay Minerals
- 29. 4.1 Origins of Charge Deficiencies Imperfections in the crystal lattice -Isomorphous substitution . The cations in the octahedral or tetrahedral sheet can be replaced by different kinds of cations without change in crystal structure (similar physical size of cations). For example , Al 3+ in place of Si 4+ (Tetrahedral sheet) Mg 2+ instead of Al 3+ (Octahedral sheet) unbalanced charges (charge deficiencies) This is the main source of charge deficiencies for montmorillonite. Only minor isomorphous substitution takes place in kaolinite.
- 30. 4.2 Origins of Charge Deficiencies (Cont.) 2 . Imperfections in the crystal lattice - The broken edge The broken edge can be positively or negatively charged.
- 31. 4.2 Origins of Charge Deficiencies (Cont.) 3 . Proton equilibria (pH-dependent charges) Kaolinite particles are positively charged on their edges when in a low pH environment, but negatively charged in a high pH (basic) environment. M M M O O - O H + H H M: metal
- 32. 4.2 Origins of Charge Deficiencies (Cont.) 4 . A dsorbed ion charge (inner sphere complex charge and outer sphere complex charge) Ions of outer sphere complexes do not lose their hydration spheres. The inner complexes have direct electrostatic bonding between the central atoms.
- 33. 4.3 “Charged” Clay Particles External or interlayer surfaces are negatively charged in general. The edges can be positively or negatively charged. Different cations balance charge deficiencies. Kaolinite and negative gold sol (van Olphen, 1991) Dry condition - or + - or + Cation
- 34. 4.4 Polar Water Molecules Structure Polar molecule H(+) H(+) O(-) Hydrogen bond Salts in aqueous solution hydration
- 35. Question Why can we use the microwave oven to cook food?
- 36. 4.5 Clay-Water Interaction Adsorbed layers 3 monolayers 1. Hydrogen bond Kaolinite Oxygen Hydroxyl Clay Surfaces Free water Bulk water The water molecule “locked” in the adsorbed layers has different properties compared to that of the bulk water due to the strong attraction from the surface. O OH H O H O H O H H
- 37. 4.5 Clay-Water Interaction (Cont.) 2. Ion hydration The water molecules wedge into the interlayer after adding water Dry condition (Interlayer) Clay layers cation The cations are fully hydrated, which results in repulsive forces and expanding clay layers (hydration energy). Na + crystal radius: 0.095 nm radius of hydrated ion: 0.358 nm
- 38. 4.5 Clay-Water Interaction (Cont.) The concentration of cations is higher in the interlayers (A) compared with that in the solution (B) due to negatively charged surfaces. Because of this concentration difference, water molecules tend to diffuse toward the interlayer in an attempt to equalize concentration. 3. Osmotic pressure From Oxtoby et al., 1994 A B
- 39. 4.5 Clay-Water Interaction (Cont.) Relative sizes of adsorbed water layers on sodium montmorillonite and sodium kaolinite Holtz and Kovacs, 1981
- 40. 5. Interaction of Clay Particles (or Layers) Interlayer Interparticle Layer Particle
- 41. 5.1 Diffuse Double Layer Clay particle with negatively charged surface x Distance x Concentration Exponential decay Cations Anions - - + + - - - - -
- 42. 5.2 Interaction Forces Net force between clay particles (or interlayers) = van der Waals attraction + Double layer repulsion (overlapping of the double layer)+ Coulombian attraction (between the positive edge and negative face) DLVO forces
- 43. 5.3 Thickness of Double Layer Thickness of double layer K K repulsion force n 0 K repulsion force v K repulsion force T K repulsion force (?) decreases with increasing temperature
- 44. 5.4 Interaction of Clay Particles Increasing Electrolyte concentration n 0 Ion valence v Temperature T (?) Decreasing Permittivity Size of hydration ion pH Anion adsorption Reduce the double layer repulsion (only applicable to some cases) Flocculated or aggregated fabric Flocculated fabric Edge-to-face (EF): positively charged edges and negatively charged surfaces (more common) Edge-to-edge (EE) Aggregated fabric Face-to-Face (FF) Shifted FF Dispersed fabric The net interparticle force between surfaces is repulsive
- 45. 5.4 Interaction of Clay Particles (Cont.) (1) Decrease pH (2) Decrease anion adsorption (3)Size of hydration Clay Particle The total required number of cations is 10 + + - - - - - - - - -
- 46. 5.5 Atterberg Limit of Clay Minerals Lambe and Whitman, 1979 Na-montmorillonite Thicker double layer LL=710 Ca-montmorillonite Thinner double layer LL=510 The thickness of double layer increases with decreasing cation valence.
- 47. 5.6 Cation Replaceability Different types and quantities of cations are adsorbed to balance charge deficiencies in clay particles. The types of adsorbed cations depend on the depositional environment. For example, sodium and magnesium are dominant cations in marine clays since they are common in sea water. In general, calcium and magnesium are the predominant cations. The adsorbed cations are exchangeable (replaceable). For example, +4CaCl 2 +8NaCl Ca Ca Ca Ca (Lambe and Whitman, 1979) Na Na Na Na Na Na Na Na
- 48. 5.6 Cation Replaceability (Cont.) The ease of cation replacement depends on the (1) Valence (primarily) Higher valence cations can replace cations of lower valence. (2) Ion size Cations with larger non-hydrated radii or smaller hydrated radii have greater replacement power. According to rules (1) and (2), the general order of replacement is Li + <Na + <K + <Rb + <Cs + <Mg 2+ <Ca 2+ <Ba 2+ <Cu 2+ <Al 3+ <Fe 3+ <Th 4+ (3) Relative amount High concentration of Na+ can displace Al 3+ . (Data compiled from Israelachvili, 1991) Cations Non-hydrated radius (Å) Hydrated radius (Å) Li + 0.68 3.8 Na + 0.95 3.6 K + 1.33 3.3 Cs + 1.69 3.3 Be 2+ 0.31 4.6 Mg 2+ 0.65 4.3 Ca 2+ 0.99 4.1 Ba 2+ 1.35 Al 3+ 0.5 4.8 Fe 3+ 0.6
- 49. 5.7 Cation Replaceability (Cont.) Hard water softener Hard water contains soluble calcium and magnesium salts such as Ca(HCO 3 ) 2 and Mg(HCO 3 ) 2 . The hardness can be removed by exchanging Ca 2+ and Mg 2+ with sodium ions Na + . For example, Na 2 Z (s) (Zeolite) + Ca 2+ (aq) CaZ (s) +2 Na + (aq) As the ion-exchange capacity of Zeolite is saturated, the capacity can be regained by passing through a concentrated solution of NaCl.
- 50. 5.7 Cation Exchange Capacity (cec) The quantity of exchangeable cations is termed the cation exchangeable capacity (cec) and is usually expressed as milliequivalents (meq) per 100 gram of dry clay ( from Mitchell, 1993) . One equivalent = 6.02 10 23 electron charges or 96500 Coulombs, which is 1 Faraday.
- 51. 5.8 Swelling Potential Practically speaking, the three ingredients generally necessary for potentially damaging swelling to occur are (1) presence of montmorillonite in the soil, (2) the natural water content must be around the PL, and (3) there must be a source of water for the potentially swelling clay (Gromko, 1974, from Holtz and Kovacs, 1981) Holtz and Kovacs, 1981 U.S. Bureau of Reclamation
- 52. 5.9 Engineering Applications Lime treatment for the swelling clay The swelling clay such as Na-montmorillonite beneath the foundation is potentially harmful to the light structure. Adding lime (CaO) into such soil can effectively reduce the swelling potential due to Ca 2+ displacing Na + , and can increase the strength by dehydration of soils and cementation. Drilling mud Soil particle The swelling clays can form a so-called “filter cake” and enable soil layers to become relatively impermeable. Earth pressure+ ground water pressure Pressure profile of slurry Trench Bentonite or Polymer Montmorillonite is the dominant clay mineral in bentonite Xanthakos, 1991
- 53. 5.9 Engineering Applications (Cont.) Xanthakos, 1991
- 54. 5.9 Engineering Applications (Cont.) Xanthakos, 1991
- 55. 5.9 Engineering Applications Dispersion agents (drilling mud; hydrometer analysis) Sodium hexa-metaphosphate (NaPO 3 ) and sodium silicate (Na 2 SiO 3 ) are used as the dispersion agent in the hydrometer analysis. How does this dispersion agent work? Three hypotheses: (1) Edge-charge reversal The anions adsorption onto the edge of the clay particle may neutralize the positive edge-charge or further reverse the edge-charge from positive to negative. The edge-charge reversal can form a negative double layer on the edge surfaces to break down flocculated structure, and assist in forming a dispersed structure. (2) Ion exchange The sodium cations can replace the divalent cations existing in the clay particles such as Ca 2+ and Mg 2+ . The decrease of cation valence can increase the thickness of the double layer and interparticle repulsi on , which can assist in forming a dispersed structure. (3) pH The higher pH may make the edge-charge tend to be negative, which can break down the flocculated structure and assist in forming a dispersed structure. The adding of dispersing agent such as sodium carbonate may slightly increase the pH.
- 56. 6. Soil Structure and Fabric
- 57. 6. Soil Structure and Fabric The structure of a soil is taken to mean both the geometric arrangement of the particles or mineral grains as well as the interparticle forces which may act between them. Soil fabric refers only to the geometric arrangement of particles (from Holtz and Kovacs, 1981). The interparticle forces (or surface forces) are relatively important for fine-grained soils at low confinement (low state of stress). “ Although the behavior of a coarse-grained soil can often be related to particle size distribution, the behavior of a fined-grained soil usually depends much more on geological history and structure than on particle size” (from Lambe and Whitman, 1979) . *Fabric and structure are used interchangeably sometimes.
- 58. 7. Soil Fabric-Natural Soil (fine-grained soils)
- 59. 7.1 Microfabric Features in Natural Soils Elementary particle arrangements , which consist of single forms of particle interaction at the level of individual clay, silt, or sand particles or interaction between small groups of clay platelets or clothed silt and sand particles. Particle assemblages , which are units of particle organization having definable physical boundaries and a specific mechanical function. Particle assemblages consist of one or more forms of elementary particle arrangements or smaller particle assemblages. Pore spaces within and between elementary particles arrangements and particle assemblages. Collins and McGown, 1974 (from Holtz and Kovacs, 1981)
- 60. 7.1 Elementary Particles Individual clay platelet interaction Individual silt or sand particle interaction Clay platelet group interaction Clothed silt or sand particle interaction Particle discernible Collins and McGown, 1974 (from Holtz and Kovacs, 1981)
- 61. 7.2 Particle Assemblages Collins and McGown, 1974 (from Holtz and Kovacs, 1981)
- 62. 7.3 Pore Space Types Collins and McGown, 1974 (from Mitchell, 1993)
- 63. 8. Soil Fabric-Clay Soils
- 64. 8.1 Terminology Dispersed: No face-to-face association of clay particles Aggregated: Face-to-face association (FF) of several clay particles. Flocculated: Edge-to-Edge (EE) or edge-to-face (EF) association Deflocculated: No association between aggregates Face (F) Edge (E) Clay Particle van Olphen, 1991 (from Mitchell, 1993)
- 65. 8.2 Particle Associations Dispersed and deflocculated Aggregated but deflocculated Edge-to-face flocculated and aggregated Edge-to-edge flocculated and aggregated Edge-to-face and edge to edge flocculated and aggregated Edge-to-edge flocculated but dispersed Edge-to-face flocculated but dispersed van Olphen, 1991
- 66. 8.3 Summary Flocculated fabric Dispersed fabric Edge-to-face (EF): positively charged edges and negatively charged surfaces (more common) Edge-to-edge (EE) The net interparticle force between surfaces is repulsive Aggregated fabric Face-to-Face (FF) Shifted Face-to-Face (FF)
- 67. 8.4 Fabric of Natural Clay Soils “ The individual clay particles seem to always be aggregated or flocculated together in submicroscopic fabric units called domains . Domains then in turn group together to form clusters , which are large enough to be seen with a visible light microscope. Clusters group together to form peds and even groups of peds. Peds can be seen without a microscope, and they and other macrostructural features such as joints and fissures constitute the macrofabric system” (from Holtz and Kovacs, 1981). Domain Cluster Ped
- 68. 8.4 Fabric of Natural Clay Soils (Cont.) Enlargement Domains and clusters with micropores Domain Cluster Ped Silt grain Micropore Macropore Yong and Sheeran (1973) (from Holtz and Kovacs, 1981)
- 69. 8.4 Fabric of Natural Clay Soils (cont.) Macrostructure , including the stratigraphy of fine-grained soil deposits, has an important influence on soil behavior in engineering practice. Joints, fissures, silt and sand seams, root holes, varves, and other defects often control the engineering behavior of the entire soils mass. The microstructure reflects the depositional history and environment of the deposit, its weathering history (both chemical and physical), and stress history. (From Holtz and Kovacs, 1981) Clay particle Water
- 70. 9. Soil Fabrics-Granular Soils
- 71. 9.1 Packing Dense packing Loose packing Honeycombed fabric Meta-stable structure Loose fabric Liquefaction Sand boil Holtz and Kovacs, 1981
- 72. 9.1 Packing (Cont.)-Sand Boil Loose sand Kramer, 1996
- 73. 9.1 Packing (Cont.) “ Contrary to popular belief, it is not possible to drown in quicksand, unless you really work at it, because the density of quicksand is much greater than that of water. Since you can almost float in water, you should easily be able to float in quicksand “( from Holtz and Kovacs, 1981 ). Help!
- 74. 9.2 Load Transfer Loading The black particles carry most of load. The remaining particles prevent the buckling of the load-carrying chains (From Santamarina et al., 2001) .
- 75. 9.3 The Relative Density (D r ) The relative density D r is used to characterize the density of natural granular soil. (Lambe and Whitman, 1979) The relative density of a natural soil deposit very strongly affects its engineering behavior. Consequently, it is important to conduct laboratory tests on samples of the sand at the same relative density as in the field ( from Holtz and Kovacs, 1981). (compaction)
- 76. Derivation
- 77. 9.3 The Relative Density (Dr) (Cont.) “ The relative density (or void ratio) alone is not sufficient to characterize the engineering properties of granular soils” (Holtz and Kovacs, 1981) . Two soils with the same relative density (or void ratio) may contain very different pore sizes. That is, the pore size distribution probably is a better parameter to correlate with the engineering properties (Santamarina et al., 2001) . 2 1 : Holtz and Kovacs, 1981
- 78. 10. Loess
- 79. Loess Loess is a type of aeolian soils, and the particles are predominantly silt-size. The soil structure is mainly stabilized by (1) the capillary force and (2) light cementation arising from the salt and fines (e.g. clay) precipitation around the contacts (Holtz and Kovacs, 1981; Santamarina, 2001). After loess is submerged, collapse of the soil structure occurs due to loss of suction and cementation Why? The interaction between water and salts and clay Capillary force Cementation Capillary force cementation
- 80. 11. Suggested Homework Read Chapter 4 Problem 4-1, 4-3, 4-4, 4-5, 4-6, 4-8(interesting)
- 81. 12. References Main References: Holtz, R.D. and Kovacs, W.D. (1981). An Introduction to Geotechnical Engineering , Prentice Hall. (Chapter 4) Mitchell, J.K. (1993). Fundamentals of Soil Behavior , 2nd edition, John Wiley & Sons (Chapter 3). Others: Israelachvili, J. (1991). Intermolecular and Surface Forces , 2 nd edition, Academic Press. Kramer, S.L. (1996). Geotechnical Earthquake Engineering , Prentice Hall. Lambe, T.W. and Whitman, R.V. (1979). Soil Mechanics , SI Version, John Wiley & Sons. Santamarina, J.C., Klein, K.A. and Fam, M.A. (2001). Soils and Waves , John Wiley & Sons. Van Olphen, H. (1991). An Introduction to Clay Colloid Chemistry . Reprint edition, Krieger Publishing Company. Velde, B. (1995). Origin and Mineralogy of Clays . Springer. Xanthakos, P.P. (1991). Surry Walls as Structural Systems, 2 nd Edition, McGraw-Hill, Inc.