15 crystallization
- 3. Crystallization Crystallization is a separation and purification technique employed to produce a wide variety of materials. Crystallization is the process by which molecules or atoms arrange themselves into definite geometrical patterns called crystals. During crystallization, phase change takes place in which a crystalline product is obtained from a solution. Importance of crystallization 1. Purification. 2. Improvement of flowability. 3. Improvement of stability. 4. Enhancement of filtration and washing. 5. Decrease of caking. 6. Improvement of appearance.
- 4. Crystallization from solution Consists of 3 steps: a. Induction of super saturation. b. Nucleation. c. Crystal growth. A] Methods of induction of super saturation: 1. Cooling: Suitable for substances whose solubility increases with temperature. It is common with inorganic salts such as KNO3 and NaNO3. 2. Solvent evaporation: Suitable for substances whose solubility is independent of temperature. It is common with salts such as NaCl and calcium acetate.
- 5. 3. Adiabatic evaporation: Suitable for thermolabile substances. It involves maintaining low pressure in a chamber containing solution which will begin to evaporate to balance lost pressure. 4. Addition of a third substance: a. It has a higher affinity to the solvent, which will result in ↓ solubility of solute in solvent salting out. b. It forms a precipitate by reacting with the solute c. It changes the pH of the solution, resulting in the precipitation of the solute.
- 6. B] Nucleation Mier's Theory: 1. A solution with temperature and concentration A is saturated by: a. cooling to temperature B b. increasing its concentration to C by evaporation. Upon further cooling or evaporation, super-saturation occurs. 2. In the metastable region (BB' or CC), there is no spontaneous nucleation. Crystallization requires the addition of external nuclei. 3. Upon further cooling or evaporation, the metastable region is exceeded and spontaneous nucleation occurs.
- 7. C] Crystal Growth The rate of crystal growth in a solution depends on: 1. Concentration gradient for transfer of solute from bulk of liquid towards the crystal face. 2. Temperature gradient for deposition of heat of crystallization at crystal face. * Heat of crystallization: Is the heat evolved or absorbed when 1 mole of a substance crystallizes. 3. Relative velocity (rotation speed) between solid and liquid.
- 8. Crystal Size 1. Fine Crystals are produced by: Rapid cooling and frequent agitation a high nucleation rate and slow rate of crystal growth. The obtained crystals are cohesive, form a cake and are difficult to wash. The purity is generally less than medium or large size crystals 2. Medium Crystals are produced by: Slow cooling without mechanical agitation.
- 9. 3. Very Large Crystals are produced by: (i) allowing large volume of solution to evaporate spontaneously or (ii) slow controlled cooling of the solution in the reaction vessel. Crystal growth occurs more slowly and subsequent filtration and washing results in the formation of perfectly pure crystals. For large crystals to grow, the solution should be perfectly clear by filtration and kept in a dust- free environment. The formation of large crystals may be facilitated by seeding.
- 10. Factors affecting crystallization process 1. Temperature ↑ temperature ↑ or ↓ solubility of the solute. KCl04 (potassium chlorate) possesses a large positive temperature coefficient of solubility (↑ temperature ↑ solubility). It crystallizes by cooling of a saturated solution. NaCl possesses a very small temperature coefficient of solubility. Crystallization by cooling is not effective. Crystallization by solvent evaporation. Sodium hydrogen phosphate and ferrous sulphate show changes in the stable form of the material with temperature. Crystallization of FeSO4< 50°C results in the formation of (FeSO4.7H2O) At higher temperature yield FeSO4.4H2O or anhydrous FeSO4.
- 11. 2. Presence of Impurities: Adsorption of impurities on the surface of the nucleolus or crystals may: a. retard rate of nucleation and crystal growth (0.1% HCl prevents the crystal growth of NaCl crystals). b. result in crystal shape modification (e.g. crystallization of NaCl in presence of urea form octahedral instead of cubic crystals) 3. Nuclei Formation Versus Crystal Growth: Growth rate is the increase in size per unit time. Nucleation rate is the number of new crystals produced per unit time. At low super-saturation, growth predominates. At higher super-saturation, nucleation predominates.
- 12. 4. Effect of Agitation: Initially, it increases crystal growth rate by: 1. ↑ heat transfer rate by ↓ thermal resistance of the boundary layer. 2. ↓ thickness and diffusional resistance of the boundary layer. At certain point, no more increase in crystal growth rate occurs. Mother liquor The liquid remaining after a crop of crystals is obtained, (generally subjected to further concentration to form crystals). * The process is repeated until recovery of all of the dissolved substances. * Crops of crystals obtained by concentration of the mother liquor are less pure than the 1st crop and require re- crystallization.
- 13. A] Cooling Crystallizers 1. Oslo cooler crystallizer Supersaturation is produced in one part of the crystallizer and is released in another. The hot concentrated solution is fed into the cooler, where super saturation to the metastable region occurs but without crystallization. A pump circulates the solution from the cooler to the tank. The supersaturated solution passes through the control pipe to the bottom of the crystallizer containing a bed of seeds (act as nuclei) where crystallization and crystal growth occurs.
- 14. Large crystals are collected from the bottom of the crystallizer by Hydraulic classification. Small crystals are formed at a higher level of solution and are removed by the cyclone separator. The mother liquor re-enters the crystallizer with the hot incoming feed and the operation is repeated. Advantages of Oslo cooler crystallizer 1. Smaller crystals can be separated from large ones. 2. No precipitation takes place in the cooler 3. Can be operated in batch & continuous modes. 4. High capacity. 5. Used to crystallize salts with a +ve temperature coefficient, e. g. NaNO3, NaClO3, KCl03 and NH4Cl Process Control Parameters: 1. Feed rate. 2. Feed temperature. 3. Heat removal in the cooler.
- 15. 2. Howard Crystallizer It is a vertical conical device through which the solution flows in an upward direction. Cooling is used to achieve super-saturation at which stage nucleation occurs. Nuclei grow as they move upward until their size is just enough to overcome gravity. At this point, the crystals settle with a uniform size. The crystal size is controlled by feed velocity.
- 16. Oslo Evaporative Crystallizer The solution is fed and heated by the heater, followed by flashing the solution in the flashing head to lose part of its solvent as vapor and become supersaturated. The supersaturated solution falls down a central pipe and up through a screen to the bed of crystals that is suspended in the crystallization chamber. B] Evaporative Crystallizers
- 17. The solution gets in contact with the suspended crystals for crystallization and crystal growth to occur. Large crystals are discharged from the bottom Fine crystals recirculate with the feed solution. This method is rapid and produces small uniform crystals. It can be used for materials with zero or negative temperature coefficient.
- 18. C] Vacuum Crystallizers They achieve super saturation by adiabatic evaporation and cooling. A hot concentrated solution enters a chamber (kept at a low pressure), the solution begins to evaporate to balance lost pressure. The latent heat of evaporation will be taken from the solution itself, which in turn becomes cold and supersaturated without external heat transfer.
- 19. Oslo vacuum crystallizer Used for thermolabile materials. Has similar design to oslo evaporative crystallizer, but without a heater. Advantages: a. Absence of heated head make it of low cost. b. Absence of cooling medium prevents the surface corrosion.
- 20. Separation of a Mixture by Extraction: Crystallization
- 21. Objectives Separation of a mixture containing an acidic and a neutral compounds by extraction. Purification of the solid component by crystallization. Identify those components from its IR spectrum and its melting point.
- 22. Terms Separation Extraction Crystallization
- 23. Separation Why we need to separate mixture?? To isolate or concentrate components from a mixture. To separate a components from other species that would interfere in the analysis
- 24. Methods of Separation Extraction Crystallization Distillation Chromatography
- 25. Extraction Extraction: Transfer of a solute from one phase to another.
- 26. Types of Extraction Can use most any combination of phases (solid, liquid, gas, supercritical fluid). Solid – Liquid - Useful for the isolation and purification of naturally occurring sources.
- 27. Extraction Making coffee is an example for extraction.
- 28. Extraction Liquid – Liquid - More common method - Depend on solubility properties of components. Like dissolves like - So ideally, the extracting solvent should be similar to the solute.
- 29. Extraction We will use two immiscible liquids. - Typically aqueous / organic solvent combos
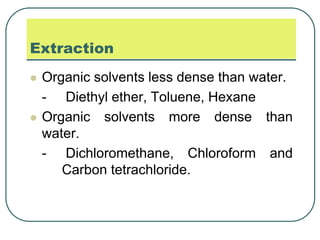
- 30. Extraction Organic solvents less dense than water. - Diethyl ether, Toluene, Hexane Organic solvents more dense than water. - Dichloromethane, Chloroform and Carbon tetrachloride.
- 31. Qualities of the Solvent Immiscible with other solvent. It should readily dissolve the compound to be extracted. It should dissolve little or none of the unwanted compounds / impurities. Easily separated from the compound. Should not undergo any reaction with the compounds.
- 32. Extraction
- 33. Extraction Equilibrium constant for this partitioning is K (partition coefficient or distribution coefficient) K= [S]2 [S]1
- 34. Extraction Chemically Inert Extraction. Chemically Active Extraction.
- 35. Chemically Active Extraction A reagent that reacts chemically with the substance to be extracted. Solubility property changes after the reaction.
- 36. Chemically Active Extraction COOH COONa Soluble in Diethyl ether & Insoluble in Water 10 % NaOH Soluble in Water & Stay in aqueous layer 10 % NaOH No Reaction & Stay in ether layer
- 37. Chemically Active Extraction NO2 CH2NH2 CH2NH3+ Soluble in Diethyl ether & Insoluble in Water 10 % HCl Soluble in Water & Stay in aqueous layer 10 % HCl No Reaction & Stay in ether layer
- 38. Procedure for Today’s Lab Get your unknown mixture. Transfer all your unknown into a 100 mL beaker and find the mass of your unknown. Add 30 mL of diethyl ether, stir slowly to dissolve the mixture. Add 15 mL more of diethyl ether and rinse the beaker.
- 39. Procedure for Today’s Lab Add 15 mL of 5 % sodium bicarbonate to the ether solution. Swirl the separatory funnel first and shake gently. Separate the two layers. Repeat these steps two times using fresh 5 % sodium bicarbonate solution. Pool all the aqueous layers i.e. sodium bicarbonate solution
- 40. Recovery of Acidic Compound Cool all the aqueous extracts for about 5 minutes in an ice – water bath. Add 3 mL of concentrated hydrochloric acid. Test the acidity of this solution with blue litmus paper (TURN RED). If it is not acidic enough; add 1 mL of acid more.
- 41. Recovery of Neutral Compound Place a small piece of cotton in a dry glass funnel. Keep a fresh and dry Erlenmeyer Flask under the funnel. Add 5g. of anhydrous sodium sulfate over the cotton plug and transfer all the ether layer carefully through the neck.
- 42. Recovery of Neutral Compound Rinse the separatory funnel with 5 mL of ether pour into drying agent. Add few boiling chips and keep on a steam bath. Cork the flask tightly once all the ether has gone.
- 43. Recrystallization It’s a technique to purify the solid organic compounds. 1. Slow evaporation 2. Slow cooling 3. Liquid diffusion 4. Use of seed crystal
- 44. Recrystallization Compound to be purified: 1. Moderate or high solubility in hot solvent 2. Low in cold solvent Impurities: 1. Insoluble in hot solvent or high soluble in cold solvent. Easily removed after crystallization. Should not react with substance being purified.
- 46. Procedure for Recrystallization Dissolve all the acidic compound in minimum amount of hot solvent. Filter the solution when it is hot. Slowly cool the solution to room temperature; then keep on ice bath. Filter the crystals, wash it with minimum amount of cold solvent. Allow to dry on its own.
- 47. Characterization Neutral Compound 1. Find the amount of neutral compound you recovered from the mixture. 2. Obtain its IR spectrum. Acidic Compound 1. Calculate the % of recovery. 2. Find its melting point.
- 48. Changes Use Blue Litmus instead of Congo Red.
- 49. Notes Do not forget to add the boiling chips when you evaporate diethyl ether. Vent the separatory funnel very often to relieve the developed pressure.
- 50. Caution NO FLAMES IN LAB TODAY
- 51. Any Questions or Additions
- 52. THANK YOU
- 53. Define the following terms: [Crystallization, Nucleation, etc] Respond to the following questions: Give a detailed account of ……………… Explain in details the process of ………….. Describe in details with examples the………… With examples, illustrate the pharmaceutical applications of ……………
- 54. Group work discussional questions: Explain in details the process of……… Describe with examples in details the………….. With examples, illustrate the pharmaceutical applications of…….



![Crystallization from solution
Consists of 3 steps:
a. Induction of super saturation.
b. Nucleation.
c. Crystal growth.
A] Methods of induction of super saturation:
1. Cooling:
Suitable for substances whose solubility increases with
temperature.
It is common with inorganic salts such as KNO3 and NaNO3.
2. Solvent evaporation:
Suitable for substances whose solubility is independent of
temperature.
It is common with salts such as NaCl and calcium acetate.](https://image.slidesharecdn.com/15-crystallization-200630110909/85/15-crystallization-4-320.jpg)

![B] Nucleation
Mier's Theory:
1. A solution with temperature and concentration A is
saturated by: a. cooling to temperature B
b. increasing its concentration to C by evaporation.
Upon further cooling or evaporation, super-saturation occurs.
2. In the metastable region (BB' or CC), there is no
spontaneous nucleation.
Crystallization requires the addition of external nuclei.
3. Upon further cooling or evaporation, the metastable
region is exceeded and spontaneous nucleation occurs.](https://image.slidesharecdn.com/15-crystallization-200630110909/85/15-crystallization-6-320.jpg)
![C] Crystal Growth
The rate of crystal growth in a solution depends on:
1. Concentration gradient for transfer of solute from bulk of
liquid towards the crystal face.
2. Temperature gradient for deposition of heat of
crystallization at crystal face.
* Heat of crystallization:
Is the heat evolved or absorbed when 1 mole of a substance crystallizes.
3. Relative velocity (rotation speed) between solid and liquid.](https://image.slidesharecdn.com/15-crystallization-200630110909/85/15-crystallization-7-320.jpg)





![A] Cooling Crystallizers
1. Oslo cooler crystallizer
Supersaturation is produced in
one part of the crystallizer and is
released in another.
The hot concentrated solution is
fed into the cooler, where super
saturation to the metastable
region occurs but without
crystallization.
A pump circulates the solution
from the cooler to the tank.
The supersaturated solution
passes through the control pipe to
the bottom of the crystallizer
containing a bed of seeds (act as
nuclei) where crystallization and
crystal growth occurs.](https://image.slidesharecdn.com/15-crystallization-200630110909/85/15-crystallization-13-320.jpg)


![Oslo Evaporative Crystallizer
The solution is fed and
heated by the heater, followed
by flashing the solution in the
flashing head to lose part of its
solvent as vapor and become
supersaturated.
The supersaturated solution
falls down a central pipe and
up through a screen to the bed
of crystals that is suspended in
the crystallization chamber.
B] Evaporative Crystallizers](https://image.slidesharecdn.com/15-crystallization-200630110909/85/15-crystallization-16-320.jpg)

![C] Vacuum Crystallizers
They achieve super saturation by adiabatic
evaporation and cooling.
A hot concentrated solution enters a chamber
(kept at a low pressure), the solution begins to
evaporate to balance lost pressure.
The latent heat of evaporation will be taken
from the solution itself, which in turn becomes
cold and supersaturated without external heat
transfer.](https://image.slidesharecdn.com/15-crystallization-200630110909/85/15-crystallization-18-320.jpg)













![Extraction
Equilibrium constant for this partitioning
is K (partition coefficient or distribution
coefficient)
K=
[S]2
[S]1](https://image.slidesharecdn.com/15-crystallization-200630110909/85/15-crystallization-33-320.jpg)



















![ Define the following terms:
[Crystallization, Nucleation, etc]
Respond to the following questions:
Give a detailed account of ………………
Explain in details the process of …………..
Describe in details with examples the…………
With examples, illustrate the pharmaceutical applications of ……………](https://image.slidesharecdn.com/15-crystallization-200630110909/85/15-crystallization-53-320.jpg)
