UDI: Where Do We Go From Here?
- 2. Healthcare Greg Bylo, GS1 US UDI: Where Do We Go From Here? GS1 US Supply Chain Visibility June 15, 2016
- 3. © 2016 GS1 US All Rights Reserved Antitrust Caution GS1 US is committed to complying fully with antitrust laws. We ask and expect everyone to refrain from discussing prices, margins, discounts, suppliers, the timing of price changes, marketing or product plans, or other competitively sensitive topics. If anyone has concerns about the propriety of a discussion, please inform a GS1 US representative as soon as possible. Please remember to make your own business decisions and that all GS1 standards are voluntary and not mandatory. Please review the complete GS1 US antitrust policy at: http://www.gs1us.org/gs1-us-antitrust-compliance-policy 2
- 4. © 2016 GS1 US All Rights Reserved Legal Disclosure GS1 US, Inc. is providing this presentation, as is, as a service to interested parties. GS1 US MAKES NO REPRESENTATIONS IN THIS REGARD AND DISCLAIMS ALL WARRANTIES, EITHER EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO, ANY WARRANTY OF ACCURACY OR RELIABILITY OF ANY CONTENT, NONINFRINGEMENT, MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. GS1 US shall not be liable for any consequential, special, indirect, incidental, liquidated, exemplary or punitive damages of any kind or nature whatsoever, or any lost income or profits, under any theory of liability, arising out of the use of this presentation or any content herein, even if advised of the possibility of such loss or damage or if such loss or damage could have been reasonably foreseen. GS1 US employees are not representatives or agents of the FDA, and the content of this presentation has not been reviewed, approved or authorized by the FDA. 3
- 5. © 2016 GS1 US All Rights Reserved Agenda 4 • Anti-Trust Caution / Legal Disclosure • Standards in Action • UDI – The Basics • Holistic Approach to UDI • Leveraging Your Investment
- 6. © 2016 GS1 US All Rights Reserved Standards in Action 5
- 7. © 2016 GS1 US All Rights Reserved GS1 Standards in Action • The following link provides a basic review of the GS1 standards for Healthcare and how they can improve operational efficiency and patient safety. • http://www.gs1us.org/industries- old/healthcare/standards-in-action-video 6
- 8. © 2016 GS1 US All Rights Reserved The Global Language of Business 7 Identify GS1 Identification Numbers Companies, Products, Locations, Logistics, Assets, and Services Capture GS1 Data Carriers Barcodes and EPC-enabled RFID Share GS1 Data Exchange Master Data, Transactional Data, and Physical Event Data
- 9. © 2016 GS1 US All Rights Reserved GS1 Standards 8
- 10. © 2016 GS1 US All Rights Reserved Implemented GS1 Standards in the Supply Chain 9
- 11. © 2016 GS1 US All Rights Reserved GS1 Interface Standards for Electronic Commerce 10
- 12. © 2016 GS1 US All Rights Reserved GS1 Interface Standards for Electronic Commerce 11 Data Flow for the Global Data Synchronization Network Select one data pool as a SINGLE point of entry to the GDSN The GS1 Global Registry® is a single repository where basic data is registered. The GS1 Global Registry identifies the data pool location of source data. Data Pools provide data that is standards conformant, and interoperable in the GDSN®. The data pool performs the transactions of sending and receiving validated product information between partners inside or outside the data pool. Step 1 : Load Data Step 2 : Register Data Step 3 : Subscription Request Step 4 : Publish Data Step 5 : Recipient Confirmation 1 2 5 5 5 4 4 3 3 3 FDA GUDID 4
- 13. © 2016 GS1 US All Rights Reserved UDI Basics 12
- 14. © 2016 GS1 US All Rights Reserved Meeting UDI Requirements • Step 1 - The Device Identification (DI) - Identify your devices - Adhere to one of issuing agencies – GS1, HIBCC, or ICCBBA 13 • Step 2 – Select Bar Code (AIDC) and design label and packaging • Step 3 – Identify the Production data •GTIN – Global Trade Item Number •Expiry Date •Lot Number •Serial Number UDI = Device Identifier (DI) + Production Identifiers (PI) GTIN + Application Identifiers (AI)
- 15. © 2016 GS1 US All Rights Reserved Meeting UDI Requirements Step 4 – The GUDID • Global Unique Device Identifier Database (GUDID) - Operated by the FDA to collect information on Medical Devices - Will have public facing website to share data with anyone - Some information submitted to the GUDID will be private (for FDA only) • All medical devices which are regulated under the UDI regulations will be required to be listed in the GUDID - Sunrise for each class is the same for GUDID as assigning a UDI • Class III devices- 1 year from final rule publication (September 24, 2014) (complete with extensions) • Class II “life sustaining” devices- 2 years from final rule publication (September 24, 2015) (GUDID extended to October 24, 2015) (complete with extensions) • Class II remaining devices- 3 years from final rule publication (September 24, 2016) • Class I devices- 5 years from final rule publication (September 24, 2018) 14
- 16. © 2016 GS1 US All Rights Reserved Holistic Approach to UDI 15
- 17. © 2016 GS1 US All Rights Reserved Implementation & Integration Implications to Consider 16 1 • MDM business process needed to maintain UDI attributes • UDI Rule provides data definitions for each attribute • Need BP Owners for each attribute 2 • Incorporate UDI into it Labeling/Packaging process • Address both global & local UDI requirements • Impacts all product labels– unit of use through case 3 • Create ECR BP to meet UDI Requirement • 48 hour requirement for design change notification • Product Design incorporate GTIN assignment process 4 • Identify all SOPs which need to be updated & include in project plan • Document integrated process in SOPs • SOPs need to be completed before project is completed 5 • Update the systems to utilize the UDI GTIN (DI) • Map product attributes from source to GUDID • Identify GUDID process & owner • Validate automated GUDID transfer process 6 • Determine sharing strategy–GDSN? • Integrate ECR process with data update process • Integrate data sharing with GUDID update process • Global and Local Approach 1 2 3 4 6 5
- 18. © 2016 GS1 US All Rights Reserved Leveraging Your Investment - Supply Chain Visibility using GS1 Standards 17
- 19. © 2016 GS1 US All Rights Reserved Critical Tracking Events 18
- 20. © 2016 GS1 US All Rights Reserved Critical Tracking Events 19 CRITICAL TRACKING EVENT DEFINITIONS TRANSFORMATION-TYPE EVENTS are those events that typically support internal traceability within the four walls of a supply chain company. TRANSFORMATION INPUT (T1) EVENT: The event where one or more materials are used to produce a traceable product that enters the supply chain.(NOTE: Materials used to produce products for immediate consumption by consumers are reported as Consumption events) TRANSFORMATION OUTPUT (T2) EVENT: The event where a traceable product is packaged and labeled for entry into the supply chain. TRANSPORTATION-TYPE EVENTS are those events that typically support external traceability between supply chain companies. SHIPPING (S) EVENT: The event where traceable product is dispatched from a defined location to another defined location. RECEIVING (R) EVENT: The event where traceable product is received at a defined location from another defined location. DEPLETION-TYPE EVENTS are those events that capture how traceable product is removed from the supply chain. CONSUMPTION (C) EVENT: The event where a traceable product becomes available to consumers. DISPOSAL (D) EVENT: The event where a traceable product is destroyed or discarded or otherwise handled in a manner that the product can no longer be used as a food ingredient or become available to consumers.
- 21. © 2016 GS1 US All Rights Reserved Key Data Elements 20 Shipping Receiving Input Output Consumption Disposal Event Type R R R R R R Event Owner R R R R R R Date R R R R R R Time R R R R R R Event Location R R R R R R Item ID Type R R R R R R Item ID R R R R R R Batch/Lot/Serial# BP* BP R R BP BP Quantity R R R R R R Unit of Measure R R R R R R Batch/Lot Relevant Date C^ C C C^ BP BP Activity Type C C R R Activity ID C C R R Supplier Identity C C C C Trading Partner Location R R R = Required Data BP = Best practice is to capture the batch/lot number for transport and depletion events whenever possible; however, if not feasible, Batch/Lot Relevant date or Activity ID must be provided. * Batch/lot/serial numbers should be reported by Suppliers for Shipping events. Transport Transformation Depletion Key Data Element C = Conditional Data; The need for this data would be determined by business circumstances; ^ Relevant Date should be reported by Suppliers for Shipping Events and for Transformation Output events.
- 22. © 2016 GS1 US All Rights Reserved GS1-128 barcode information for Visibility • When using a GS1-128 barcode, Application Identifiers (AI’s) are encoded for scanning purposes. AI’s are flags that precede each piece of data in a barcode to identify its meaning and format. • GS1-128 provides the ability to string together additional fields of information: − (01) precedes a GTIN ( 00614141987658 ) − (11) precedes Production Date (YYMMDD) (120715) − (10) precedes Batch/Lot Number ( ABC123 ) 21
- 23. © 2016 GS1 US All Rights Reserved How do we Share this data? 22
- 24. © 2016 GS1 US All Rights Reserved Visibility Data Content 23 Visibility data consists of events, each of which records something that happened in the real world triggered by reading a barcode or an RFID tag. An event has four dimensions: - What: what physical objects were involved (EPC) - When: when the event took place (timestamp) - Where: where the event took place (location identifier) - Why: what business process step was being carried out Where and Why are what distinguish visibility data useful to a business application from raw RFID or Bar Code data
- 25. © 2016 GS1 US All Rights Reserved The “What” Dimension 24 1732050807+ Company Code Product Code Lot/Batch or Serial Number • Tells you: - What product (GTIN / U.P.C.) - What instance (lot/batch or serial number)
- 26. © 2016 GS1 US All Rights Reserved The “Where” Dimension 25 • A location identifier that says - Where the event took place; and/or - Where things are following the event • Understood by applications - Master data synchronization important! • Meaningful - E.g., two rooms separated by a door, report which room, not the location of the door • Not necessarily the name of the reader - E.g., for a bar code / RFID-enabled forklift, indicate into which bin the load was dropped, not which forklift did it • Location sensor, location tag, etc – not the reader identity Room 1 Room 2
- 27. © 2016 GS1 US All Rights Reserved The “Why” Dimension 26 • Identify the business context of the visibility event: - What business step was taking place at the time of the event - What is true from a business perspective after the event - Any associated business transactions • Purchase Order, Invoice, BOL, etc Ski #23 Tue 10:05 Rent Shop Checkout Rented Ski #23 Tue 10:15 Base Rack Observe Ski #23 Tue 10:20 Lift #1 Observe Ski #23 Tue 10:23 Summit #1 Observe Ski #23 Tue 11:05 Rent Shop Checkin Available Ski #23 Tue 13:08 Repair Begin Repair Ski #23 Tue 13:10 Rent Shop Done Repair Ski #23 Tue 13:06 Base Rack Observe Ski #23 Tue 13:07 Rent Shop Problem Ski #23 Tue 13:05 Rent Shop Checkout Rented Rental Contract #1235 John Doe Rental Contract #1325 Jane Roe
- 28. © 2016 GS1 US All Rights Reserved Visibility in the Supply Chain 27 Pack Line Ship Door Ship Door Rcv Door Interior Door Trash Compactor R1 R2 R3 R4 R5 R6 R7 DC1 - Mfr DC2 - Retailer Store1 Rcv Door Back Front Promotion / NPI Tracking Supply Routes Analysis Real-time Inventory Correction EPCIS Visibility Data Product Recall Execution Demand Planning Response
- 29. © 2016 GS1 US All Rights Reserved Visibility Architecture 28 EPCIS Visibility Data Business Applications Data Capture Infrastructure R R R R Single Most Important Design Decision: This Interface Key “hinge” between different worlds Enterprise Software Factory Automation Overall Business Process Single Material Handling Step IT/LOB Operations/ Labor
- 30. © 2016 GS1 US All Rights Reserved Example – Forward Deployed Inventory 29 Implant #1 Tue 10:05 Mfg. Plant 1 Goods Receipt Implant #1 Tue 1:05 Mfg. Plant 1 Shipment Implant #1 Tue 4:30 DC #1 Goods Receipt Implant #1 Wed 12:10 Replenish Store #2 Goods Receipt Tray #10 Thur 12:05 Replenish Store #2 Aggregate Implant #1 Wed 8:30 DC #1 Shipment Tray #10 Thur 3:00 Replenish Store #2 Shipment Tray #10 Sat 9:00 Hospital #1234 Shipment Tray #10 Fri 9:15 Hospital #1234 Goods Receipt Tray #10 Tue 1:05 Hospital #1234 Goods Receipt Implant #1 Fri 3:30 Hospital #1234 Consumption
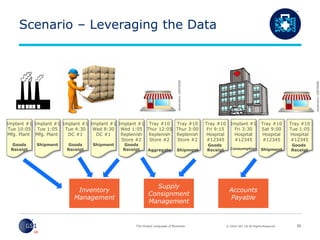
- 31. © 2016 GS1 US All Rights Reserved Scenario – Leveraging the Data 30 Implant #1 Tue 10:05 Mfg. Plant Goods Receipt Implant #1 Tue 1:05 Mfg. Plant Shipment Implant #1 Tue 4:30 DC #1 Goods Receipt Implant #1 Wed 1:05 Replenish Store #2 Goods Receipt Tray #10 Thur 12:05 Replenish Store #2 Aggregate Implant #1 Wed 8:30 DC #1 Shipment Tray #10 Thur 3:00 Replenish Store #2 Shipment Tray #10 Sat 9:00 Hospital #12345 Shipment Tray #10 Fri 9:15 Hospital #12345 Goods Receipt Tray #10 Tue 1:05 Hospital #12345 Goods Receipt Implant #1 Fri 3:30 Hospital #12345 Consumption Inventory Management Supply Consignment Management Accounts Payable
- 32. © 2016 GS1 US All Rights Reserved Data Sharing with Known Trading Partners 31 Supplier Retailer Factory Distribution Ctr Distribution Ctr Retail Stores EPCIS Database EPCIS Database 1. Visibility data collected during tagging and shipping 2. Visibility data collected as product moves 3. Retailer data shared with supplier via retailer’s network 4. Combined data used to gain business benefits
- 33. © 2016 GS1 US All Rights Reserved Focus on Data Interfaces – Maximum Flexibility 32 Visibility-Driven Business Applications Visibility Data Hub Visibility Data Capture (1 or more sites) Visibility Data Definitions (matrix) Governance Master Data Configuration
- 34. © 2016 GS1 US All Rights Reserved Macy’s Inc. - Driving Inventory Accuracy & Omni-Channel Fulfillment 33 Macy’s, Inc. is one of the nation’s premier omni- channel retailers. As of February 23, 2016, the company operates about 870 stores in 45 states, the District of Columbia, Guam and Puerto Rico. Macy’s, Inc.’s diverse workforce includes approximately 166,900 employees. macysinc.com Situation Approach In late 2011, Macy’s embarked on a 3-year omni- channel excellence journey. Looking to increase color/size inventory visibility and to drive sales in their high replenishment categories, They began using EPC-enabled RFID technology to execute more frequent cycle counts and improve sampling compliance in women’s and men’s shoes. GS1 Standards support: • GS1 EPC Radio-Frequency Identity Generation- 2 UHF RFID Protocol (GS1 SGTIN-96 coding schema for these tags) • GS1 US Apparel and General Merchandise Initiative membership • Workgroup participants - Macy’s has collaborated with industry trading partners in developing industry best practices and driving industry adoption. Since 2011, their adoption of RFID has evolved from a transformational technology to a foundational requirement for inventory optimization and has grown to all 800+ Macy’s and Bloomingdale’s stores. They expanded RFID use from replenishment to fashion areas and saw their sales volumes increase more than 200%. In 2014, they completed their single view of inventory system upgrades. Macy’s now has one “view” of inventory— accurate inventory through RFID—with a focus on optimizing in-stock inventory to satisfy customer demand. Converting to a single view inventory was enabled by leveraging many GS1 Standards including, but not limited to: GTIN, SSCC-18, EDI, and EPC/RFID. Results & Benefits 3-Yr Results: • Inventory optimization in their omni-channel fulfillment processes (freeing up the last one (GTIN) for sale … instead of working with thresholds and minimum units/location before making it available for sale via their e-com channel) • Sustaining a 95% item accuracy at the size/color level (improved from 75%) • Selling floor shoe sample compliance growth (from 70% style representation to 95%+) • Sales trends increases in RFID tagged product categories (increasing in the high single digits to low double digits vs the non-RFID comparable control groups) Benefits ~ Previously, store and online assortments were bought and marketed by separate organizations at Macy’s and at Bloomingdale’s. These changes enable Macy’s to: • Continue to support organizational growth & the enhanced unified shopping experience • Have an accurate inventory file to drive customer demand & better business decisions • Increase sales & customer satisfaction through improved in-store availability and enhanced omni- channel fulfillment (e.g., buy online and ship from / pick up in store).
- 35. © 2016 GS1 US All Rights Reserved Macy’s Inc. - Driving Inventory Accuracy & Omni-Channel Fulfillment • Their RFID-enabled departments outperformed controls by 10% (between Sept. 2013 and May 2014) and their display rates improved from 70% to 95%. 1 • Further, demonstrating that RFID enables better “last-item” visibility and the ability to sell that item at the higher full margin price point, Macy’s CEO, Terry Lundgren reported that their “buy online/pick-up in-store results totaled 125% of intended order.” 2 1. Retail Touch Points Webinar, Sept 16, 2014, “Omni-channel Leaders Reaffirm The Value Of RFID” 2. Marketing Daily, Sept 3, 2014, “Macy's Focused On Mobile, Gen Y, Private Label” 34
- 36. © 2016 GS1 US All Rights Reserved Wrap Up • Determine what “challenges” visibility can solve in your organization - Inventory Utilization? - Supply Management – shortages? • Validate process flows are really what is happening • Do a risk analysis of the events in the process flow to determine when to capture the data and how long to maintain it • Are all systems enabled for the process? • Apply learnings to other parts of your business 35
- 37. © 2016 GS1 US All Rights Reserved Supply Chain Visibility Workshop • The below link takes you to a GS1 US website dedicated to supply chain visibility. Additional information and workshops are available to support an organization who wants to pursue this further. - https://www.gs1us.org/about-gs1-us/events/supply-chain- visibility 36
- 38. © 2016 GS1 US All Rights Reserved T E www.gs1us.org GS1 US Corporate Headquarters Princeton Pike Corporate Center 1009 Lenox Drive, Suite 202 Lawrenceville, NJ 08648 USA Contact Information 35 Greg Bylo VP Healthcare Office (609) 620-8073, Cell (609) 216-4346